zh-TW
在導航的名稱

The annual cycle of antler growth in O. hemionus is initiated and controlled by changes in day length acting on several cell types of the anterior pituitary. These cell types secrete growth-stimulating hormones that act mainly on the antlers and incidentally on the testes. Antler hardening, shedding, and the breeding period are mediated by decreasing day length through the action of gonadotropins on Leydig cells, thus producing androgens. Androgens induce secondary ossification, accelerate maturation, induce behavioral changes that result in shedding antler velvet, and aid in the maintenance of osteoblasts and osteocytes to maintain antlers in hard bone condition. Withdrawal of androgens at the end of the breeding season permits resorption of bone at the pedicel-antler junction and antler shedding. O. hemionus has excellent binocular vision. While unable to detect motionless objects, O. hemionus is extraordinarily sensitive to moving objects. The sense of hearing is also extremely acute. O. hemionus is a target for various viral, bacterial, and parasitic diseases. For example, heavy amounts of gastrointestinal nematodes may cause death in O. hemionus. This parasitic disease is usually indicative of such predisposing factors as high mule deer density and malnutrition. Infection by the parasitic meningeal worm can cause fatal neurologic disease in O. hemionus. Livestock may transmit viral diseases to O. hemionus as seen in foot-and-mouth disease. This infection is characterized by blisters in the mouth, above the hooves, and between the digits (Anderson 1984).
Perception Channels: tactile ; chemical
All federal, state, and provincial land and wildlife management agencies recognize the fundamental need to maintain O. hemionus ranges and keep them habitable. To counter the trend of agricultural development, rangeland conversion, mining, road and highway construction, and the development of housing tracts, many states and provinces have purchased critical areas, especially winter ranges, to maintain the various habitats of O. hemionus. But, due to political opposition to government acquisition of privately owned lands, plus a scarcity of funds for this purpose, only a small fraction of O. hemionus ranges has been acquired by the government. The effects of reduced O. hemionus ranges can be mitigated by better management of the remaining lands to maximize their productiviy for O. hemionus. Various habitat management programs include the manipulation of livestock grazing, the manipulation of cultivative communities, and the manipulation of vegetative communities. For O. hemionus, the optimal successional stages are subclimax plant communities that can be perpetuated only through the influence of humans. Since O. hemionus production is not the primary management goal on most private or public lands in western North America, O. hemionus habitat improvement programs typically involve a complex process of coordination among bureaucracies with missions that are usually not compatible (Wallmo 1981).
IUCN Red List of Threatened Species: least concern
Douglas fir and Ponderosa pine are of major economic importance for commercial timber. However, these trees are browsed heavily by O. hemionus. Browsing of other trees is seldom considered an economic problem. In the Douglas fir region, O. hemionus browses on trees during both the dormant and growing seasons. Practices that encourage the growth of O. hemionus populations can therefore also encourage damage. Douglas fir is harvested mainly by clearcutting and is regenerated by planting with nursery-grown stock. O. hemionus is attracted to clear-cuts, and Douglas fir is an acceptable and sometimes preferred forage species. This situation invites browsing of sufficient intensity to influence forest regeneration in many areas (Wallmo 1981).
Odocoileus hemionus is of tremendous interest to hunters. Populations of O. hemionus that are large enough to support hunting during two or three weeks in autumn offer countless recreational opportunities for the public. This desire to hunt generates revenue for the economy (Wallmo 1981).
Odocoileus hemionus is a small ruminant with limited ability to digest highly fibrous roughage (Short 1981). Optimum growth and productivity of individuals and populations are dependent upon adequate supplies of highly digestible, succulent forage. Diets consisting primarily of woody twigs cannot meet the maintenance requirements of O. hemionus. Based on its stomach structure and its diet of woody and herbaceous forage in approximate equal proportions, O. hemionus is classified as an intermediate feeder. Because nutritious forage is in poor supply for much of the year, O hemionus has an annual cycle of metabolic rates. A higher energy flux and food intake in the summer enables O. hemionus to capitalize on abundant high-quality forage for growth and fat storage. A lower energy flux in the winter permits O. hemionus to survive on a lower intake of poor-quality forage while minimizing the catabolism of stored fat for body functions. The estimated rate of food intake is about 22 g/kg body weight/day. In adult males, food intake drops abruptly with the onset of rut (Anderson 1984). O. hemionus frequently browses leaves and twigs of trees and shrubs. Green leaves are very succulent and, except for epidermal tissue and structural ribs, consist largely of easily digestible cell contents. Dead and weathered leaves have little protein and high cell-wall values. As a result, they are of very low digestibility. O. hemionus also eats acorns, legume seeds, and fleshy fruits, including berries and drupes, that have moderate cell-wall levels and are easily digested (Short 1981).
Odocoileus hemionus occurs over most of North America west of the 100th meridian from 23 degrees to 60 degrees N. The eastern edge of the usual range extends from southwestern Saskatchewan through central North and South Dakota, Nebraska, Kansas, and western Texas. Isolated occurrences have been reported from Minnesota, Iowa, and Missouri. Major gaps in geographic distribution are in southern Nevada, southeastern California, southwestern Arizona, and the Great Salt Lake desert region. Apart from these gaps, O. hemionus occurs in all of the biomes of western North America north of central Mexico, except the Arctic tundra (Anderson 1984).
Biogeographic Regions: nearctic (Native )
Odocoileus hemionus is remarkably adaptable. Of at least sixty types of natural vegetation west of the 100th meridian in the United States, all but two or three are or once were occupied by O. hemionus. Several additional vegetation types are inhabited in Canada and Mexico as well. The vegetation types in Mexico are similar to the types occurring in the United States. However, the tropical deciduous vegetation at the tip of Baja California is unique. In Canada, O. hemionus occupies five boreal forest types that do not occur in the United States. O. hemionus occupies a wide range of habitat provinces (regions of land containing particular vegetation types) in western North America. These habitat provinces include the California woodland chaparral, the Mojave Sonoran desert, the Interior semidesert shrub woodland, the Great Plains, the Colorado Plateau shrubland and forest, the Great Basin, the Sagebrush steepe, the Northern mountain, and the Canadian boreal forest (Wallmo 1981).
Terrestrial Biomes: desert or dune ; savanna or grassland ; chaparral ; forest ; mountains
Average lifespan
Status: captivity: 22 years.
The pelage of Odocoileus hemionus ranges from dark brown gray, dark and light ash-gray to brown and even reddish. The rump patch may be white or yellow, while the throat patch is white (Geist 1981). The white tails of most mule deer terminate in a tuft of black hairs, or less commonly in a thin tuft of white hairs. On some mule deer, a dark dorsal line runs from the back, down the top of the tail, to the black tail tip. All markings vary considerably among O. hemionus, but remain constant throughout the life of an individual. O. hemionus possess a dark V-shaped mark, extending from a point between the eyes upward and laterally. This mark is more conspicuous in males. Growth in O. hemionus during the first year is roughly parallel in males and females. Thereafter, males, in general, exceed females in carcass weight, chest girth, neck circumference, body length, head length, cranial breadth, shoulder height, hindfoot length, and hoof length (Anderson 1984). Carcass weight ranges from 45 to 150 kg in males, and 43 to 75 kg in females. Chest girth ranges from 80 to 117 cm in males, and 78 to 97 cm in females. Neck circumference ranges from 30 to 65 cm in males, and 26 to 38 cm in females. Body length ranges from 126 to 168 cm in males, and 125 to 156 cm in females. Head length ranges from 28 to 35 cm in males, and 27 to 33 cm in females. Cranial breadth ranges from 11 to 16 cm in males, and 10 to 14 cm in females. Shoulder height ranges from 84 to 106 cm in males, and 80 to 100 cm in females (Wallmo 1981).
Range mass: 43 to 150 kg.
Other Physical Features: endothermic ; bilateral symmetry
Odocoileus hemionus is a polygynous species, having a tending-bond type breeding system. Courtship and mating occur within the group (Geist 1981). A dominant male tends an estrus female until mating or displacement by another male occurs. Dominance is largely a function of size, with the largest males, which possess the largest antlers, performing most of the copulations (Kucera 1978). Most O. hemionus females conceive during their second year and only rarely during their first year. The breeding peak in O. hemionus occurs mainly from late November through mid-December. The average gestation length is 204 days. The peak birth period in O. hemionus is estimated to be from June 16th to July 6th, with most births occurring in June. The time of birth varies according to the environment. Robinette (1977) calculated that a 305-m rise in elevation is associated with a 7-day delay in the birth period. The mass at birth of O. hemionus ranges from 2 to 5 kg. Mass at birth is affected by litter size and sex, with males being heavier. The common liter size is two, with mothers in their first or second breeding year most frequently producing singletons. Weaning begins at about 5 weeks of age and usually is completed at age 16 weeks. Full development of most skeletal attributes occurs at about 49 months of age in males and 37 months of age in females. However, gains in carcass mass are continuous until an age of 120 months in males and 96 months in females. In O. hemionus, male neonates predominate when poor nutrition prevails about 6 weeks before, and during, the breeding period. Ovulation in female O. hemionus occurs about 12 to 14 hours after estrus terminates. Approximately 27 to 29 days elapse between conception and implantation in female O. hemionus. Among male O. hemionus, testicular mass and volume are maximal during November and minimal during April and May (Anderson 1984).
Key Reproductive Features: gonochoric/gonochoristic/dioecious (sexes separate); sexual
Average birth mass: 2950 g.
Average gestation period: 207 days.
Average number of offspring: 1.5.
Average age at sexual or reproductive maturity (male)
Sex: male: 503 days.
Average age at sexual or reproductive maturity (female)
Sex: female: 478 days.
Mule deer are the most widely distributed and abundant of all large mammal species in western North America. They occur in diverse habitats from moist, dense coniferous forests to dry, open plains and deserts, and alpine habitats [129]. A review stated that "the multitudinous habitats of the mule and black-tailed deer are so diverse as to defy generalization" [347]. Mule deer occur in tallgrass, mixed-grass, and shortgrass prairies of the Great Plains, in shrublands, woodlands, and forests of the Rocky Mountains, and in sagebrush (Artemisia spp.) communities, pinyon-juniper (Pinus-Juniperus spp.) woodlands, and desert scrub of the Southwest. They are absent, however, from many desert communities of the Southwest because succulent forage occurs too infrequently to maintain populations [347]. In terms of elevation, they occur from coastal communities up to subalpine and alpine communities [119,215,347]. See the Fire Regime Table for a list of plant communities in which mule deer may occur and information on the FIRE REGIMES associated with those communities.
Canadian boreal forest: Mule deer occur in boreal forests of the 4 major forest types: quaking aspen (Populus tremuloides) parkland; mixed woodlands of quaking aspen, balsam poplar (P. balsamea), paper birch (Betula papyrifera), resin birch (B. glandulosa), white spruce (Picea glauca), and balsam fir (Abies balsamea); lower foothills dominated by lodgepole pine (Pinus contorta), quaking aspen, and balsam fir with white spruce and black spruce (Picea mariana); and northern foothills of black spruce, white spruce, subalpine fir (A. lasiocarpa), and pine (Pinus spp.). The boreal forest-subarctic woodland ecotone marks the northern limits of mule deer's distribution. In the boreal forest ecosystem, mule deer prefer open grassland-parkland types [347]. In Yukon, mule deer habitats are largely open, south-facing grassy slopes bordered by quaking aspen, and recently burned areas. Mule deer expanded their natural range into Yukon around the 1930s [152].
Alaska, Pacific Northwest, and California: In Alaska, black-tailed deer occur in Sitka spruce (Picea sitchensis), western redcedar (Thuja plicata), and western hemlock (Tsuga heterophylla) forests. Inland these forests transition to alpine tundra [135]. In coastal regions of northern California and southern Oregon, redwood (Sequoia sempervirens) and Douglas-fir (Pseudotsuga menziesii) dominate black-tailed deer habitats. To the north, throughout habitats in Washington, British Columbia, and Alaska, Sitka spruce, western redcedar, and western hemlock dominate [135,215,347]. Inland in northern California, Oregon, Washington, and British Columbia, black-tailed deer occur in western redcedar, incense-cedar (Calocedrus decurrens), western hemlock, and/or Douglas-fir forests. On the Coast Ranges, they occur in Douglas-fir and silver fir (A. alba) forests [135]. In the Cascades Range, silver fir-Douglas-fir, subalpine fir-mountain hemlock (T. mertensiana), and ponderosa pine (Pinus ponderosa)-shrub forests are common mule deer habitats, whereas in the Sierra Nevada, mule deer commonly occur in mixed-conifer forests (white fir (A. concolor), incense-cedar, sugar pine (P. lambertiana), ponderosa pine, and Douglas-fir), red fir (Abies magnifica) forests, and subalpine forests (lodgepole pine, whitebark pine (P. albicaulis), foxtail pine (P. balfouriana), and/or mountain hemlock) [347,348]. Throughout coastal and inland California, mule and black-tailed deer occur in mixed-evergreen forests comprised of incense-cedar, Pacific madrone (Arbutus menziesii), California bay (Umbellularia californica), Coulter pine (P. coulteri), canyon live oak (Quercus chrysolepis), and/or coast live oak (Q. agrifolia) [52,347]. Throughout California, mule deer are particularly common in oak woodlands and chaparral. California oak woodlands are dominated by a mixture of oaks including coast live oak, canyon live oak, blue oak (Q. douglasii), valley oak (Q. lobata), and interior live oak (Q. wislizeni), and pines such as gray pine (Pinus sabiniana) and Coulter pine [347]. California chaparral communities occupied may be monospecific communities dominated by chamise (Adenostoma fasciculatum), manzanita (Arctostaphylos spp.), or ceanothus (Ceanothus spp.) or diverse mixtures with oaks (Quercus spp.) and other shrubs [340,347]. From California north to Washington and east to Wyoming, mule deer occur in sagebrush steppe. Antelope bitterbrush (Purshia tridentata) and snowberry (Symphoricarpos spp.) are important shrubs browsed by mule deer in this ecosystem and may be codominant in some areas [287,347].
Southwest: Mule deer occur in a range of habitats in the Southwest, including desert shrublands at the lowest elevations, semidesert shrubland-grassland communities, chaparral, mountain shrub, woodlands at midelevations, and some forests at high elevations [347]. Desert grasslands without shrubs do not have mule deer unless they contain rugged topography or riparian areas. Dry washes are important to mule deer in semidesert grasslands because they provide food as well as resting, escape, and travel cover throughout the year. In parts of the Mojave, Sonoran, and Chihuahuan deserts, mule deer are restricted almost entirely to riparian habitats [290]. In the Sonoran Desert, they are most abundant on upper bajadas in desert scrub either in or near ecotones with interior chaparral, grassland, or woodland [340]. Interior Arizona chaparral provides good year-round habitat for mule deer [290,345].
Great Basin: In the Great Basin, mule deer occur in semidesert shrublands of sagebrush, saltbush (Atriplex spp.), Stansbury cliffrose (Purshia mexicana var. stansburiana), and winterfat (Krascheninnikovia lanata) [215,347]. At high elevations, mule deer occur in juniper-pinyon woodlands and in forests of lodgepole, ponderosa, Great Basin bristlecone (P. longaeva), and/or limber (P. flexilis) pine [101,347]. Great Basin conifer woodland [53,290] and Great Basin montane scrubland [54] also provide good habitat for mule deer. Pinyon-juniper woodlands are used as year-long mule deer rangeland but are particularly valuable as winter rangeland [290,305]. Montane and subalpine conifer forests, particularly those near mountain meadows or quaking aspen communities, serve primarily as summer rangelands [51,290].
Rocky Mountains: In northern mountainous areas of the West, montane and subalpine forest communities dominate summer ranges and open, shrub-dominated slopes and ridges are the primary winter rangeland [215]. Forests of quaking aspen, grand fir (A. grandis), western larch (Larix laricina), western white pine (P. monticola), western redcedar, and/or western hemlock, meadows, and alpine communities are common mule deer habitats. In valleys and piedmonts of the Rocky Mountain region, grasslands and open ponderosa pine forests are common mule deer habitats [347,348]. Sagebrush steppe, juniper-pinyon woodland, and true mountain-mahogany (Cercocarpus montanus)/oak scrub are the most extensive winter rangeland types in northern mountainous regions [66,348].
Mule deer use a wide variety of habitats in southern mountainous areas. The "most significant" are sagebrush, juniper-pinyon woodland, mountain shrub, montane forest, and subalpine forest. At low elevations, big sagebrush (A. tridentata), juniper (e.g., oneseed (Juniperus monosperma), Utah (J. osteosperma), alligator (J. deppeana), and Rocky Mountain juniper (J. scopulorum)), and pinyon occur in various combinations. The true mountain-mahogany/oak shrub type and many pine and Douglas-fir types occur at midelevations. In subalpine forests, subalpine fir, blue spruce (P. pungens), and Engelmann spruce (P. engelmannii) are most prevalent to the south and lodgepole pine, subalpine fir, and Engelmann spruce are most prevalent to the north. Mule deer occur in quaking aspen habitats in montane and lower subalpine zones [347].
Great Plains: In the prairies of the northern United States and southern Canada, rough, forested, or nonforested breaks along river drainages, badlands, and shrubby stream courses and draws—especially near agricultural lands—provide mule deer habitat. Level and rolling grasslands provide poor habitat for mule deer [215,287,347]. Mule deer populations diminish abruptly at the transition from shortgrass or mixed-grass prairie to tallgrass prairie [347]. Mule deer occur in the quaking aspen parklands of Alberta and Saskatchewan [287]. Mule deer prefer shrubby draws dominated by western snowberry (S. occidentalis), common snowberry (S. albus), silver buffaloberry (Shepherida argentea), chokecherry (Prunus virginiana), golden currant (Ribes aureum), and rose (Rosa spp.); hardwood draws dominated by green ash (Fraxinus pennsylvanica), American elm (Ulmus americana), boxelder (Acer negundo), and hackberry (Celtis occidentalis); and floodplains dominated by eastern cottonwood (Populus deltoides) with willow (Salix spp.) and saltcedar (Tamarix spp.) in the understory. Slopes with Rocky Mountain juniper are also important [287,288]. In Prairie County, Montana, over all seasons and years, mule deer generally used sagebrush-grasslands, bunchgrass prairies, badlands, mesic shrublands, and hardwood draws more than expected based on availability [365].One review described mule deer as polygamous, with bucks wandering about extensively and seeking individual does in estrus [215]. Other reviews described them as polygynous [7,119], with a tending bond system where a doe seeks out a dominant buck and the buck tends to the doe until she is bred [7,119,121]. The largest bucks with the largest antlers are dominant and breed most often [7,215].
The breeding season (rut) begins as early as September and ends as late as March, depending upon location [7,215]. Within a given location, however, breeding tends to occur within a short period [7]. For example, in the Missouri River Breaks region of eastern Montana, 75% of pregnant does conceived between 21 November and 1 December [129]. A single buck may breed many females, and a single doe may breed several times during a single estrous period [58,215]. Yearling (1- to 1.5-year-old) females tend to breed 3 to 4 weeks later than adults [14,102].
The interval between estrous periods ranges from 22 to 29 days, although in black-tailed deer the estrous cycle may shorter. True estrus lasts 24 to 36 hours [5,7,58,215]. As many as 5 estrous periods may occur when does repeatedly fail to conceive [7,102].
Slope, aspect, and elevation influence mule deer habitat use, particularly through effects on snow depth [135] and ambient temperature [327]. In general, high elevations tend to have more snow than low elevations, and south- and west-facing slopes tend to have less snow than other aspects due to solar radiation. Steep south-facing slopes are probably useable longer in fall, winter, and spring than shallow south-facing slopes or any north-facing slope. Shading, wind direction, and other topographical features are also important [66,135].
Figure 3. Mule deer herd on a snowy slope. Photo courtesy of David Heffernan, USFWS.During hot weather, mule deer in the Southwest tend to forage on north- or west-facing slopes in dense vegetation, to bed in shade, and to seek shelter in washes [290]. In Lake County, California, black-tailed deer use mostly south-facing slopes in winter. In late spring they use mostly cool, northern exposures until fall, although they sometimes move to streambeds during hot weather and use south-facing slopes at night. They also move up and down elevation throughout the day and year, using areas near ridgelines in cool weather and deep canyon bottoms during hot weather [14,327]. In Fort Bayard, New Mexico, mule deer used all slopes throughout the year. The author concluded that a diversity of slopes and aspects likely benefited mule deer by providing diverse forage and protection from weather [157].
Along the continuum from grazers to browsers, mule deer are classified as intermediate or mixed feeders and can switch from a diet composed primarily of grasses and forbs to one primarily of browse [7,119,216]. Mule deer are opportunistic, concentrate selectors. Compared with other ruminants, they have small rumens and gut lengths relative to body size; thus, they must eat small volumes of high-quality, easily digested food [121,192,215]. Mule deer consume the stalks, flowers, fruits, and seeds of grasses and forbs. They eat the buds, fruits, seeds (particularly acorns), stems, leaves, and bark of trees and shrubs [88,119,215,293]. They also eat fungi, lichens, algae, mosses, and ferns [135,185,215,293,340]. Cacti and other succulents may be seasonally important in mule deer diets in some areas [215,340]. Mule deer may eat aquatic vegetation, but according to Cowan [81], they do not normally feed in water >8 inches (20 cm) deep. Mule deer can only access forage that is <5 feet (1.5 m) tall [119,145,327]. They avoid dense thickets when feeding [81,102,141,145,181,327]. Reviews of mule deer diets are available: [88,185,340,348].
The foods eaten by mule deer are extremely varied [119]. For example, a review listed 202 species of trees and shrubs, 484 species of forbs, and 84 species of graminoids eaten by Rocky Mountain mule deer [185], which occur from Yukon and Alberta south to Arizona and Texas [185]. In the Southwest, a review of 12 studies across Arizona, New Mexico, and southwestern Texas found that 327 plant species were consumed by mule deer [290]. Although mule deer utilize a large number of species, relatively few make up a large part of their diet. For example, in Colorado pinyon-juniper woodland in Fort Bayard, New Mexico, tame mule deer sampled 113 of the 194 plant species found in the area, but only 10 comprised ≥92% of the diet [157].
Forbs and grasses are the most important mule deer forages during the growing season in most regions, whereas browse is often most important during the dormant season [41,42,88,101,241,290,327,330,340]. In the coastal forests of southern Vancouver Island, mule deer annual diets consisted of 67% browse, 15% lichens (mostly beard lichens (Usnea spp.)), 11% forbs, 5% fungi, and 2% graminoids, ferns, horsetails (Equisetum spp.), and quillworts (Isoetes spp.). Mule deer fed on 92% of available browse species, 64% of forbs, 56% of graminoids, and 74% of ferns, horsetails, and quillworts throughout the year [81]. In conifer forest in the North Coast Ranges of California, browse, including acorns, were eaten consistently throughout the year (48% of annual diet), forbs were eaten mostly in summer (28% of annual diet), and graminoids were important in cool months (24% of annual diet) (California Wildlife Investigations Laboratory cited in [88]). Forb quality and quantity appeared to increase the productivity of 2 mule deer herds in Utah. On a summer range with forage that was 52% forbs, a herd averaged greater carcass weights and greater fawn production than a herd on a range where only 12% of forage was forbs [250].
Typical genera browsed by black-tailed deer along the northern Pacific Coast include maple (Acer spp.), alder (Alnus spp.), wintergreen (Gaultheria spp.), currant (Ribes spp.), blackberry (Rubus spp.), willow (Salix spp.), elderberry (Sambucus spp.), and huckleberry (Vaccinium spp.) [324]. "Staple" forage plants for black-tailed deer in California chaparral include chamise, interior live oak, and wedgeleaf ceanothus (Ceanothus cuneatus) [36]. Oak foliage and acorns are important in regions where they occur [10,36,48,57,236,327]. In the Great Basin, antelope bitterbrush, mountain-mahogany (Cercocarpus spp.), sagebrush, and juniper are major components of the mule deer's winter diet [41,42,290,348,354]. Other important browse plants include ceanothus, manzanita, serviceberry (Amelanchier spp.), desert peach (Prunus andersonii), and rose [41]. Forage species important to Rocky Mountain mule deer are shown below [185].
Table 1. Forage species most valuable to Rocky Mountain mule deer in at least one season [185]
Browse antelope bitterbrush big sagebrush chokecherry curlleaf mountain-mahogany (Cercocarpus ledifolius) Gambel oak (Quercus gambelii) hollyleaved barberry (Mahonia aquifolium) ponderosa pine quaking aspen rabbitbrush (Chrysothamnus spp.) Rocky Mountain juniper rose Saskatoon serviceberry (Amelanchier alnifolia) snowberry skunkbush (Rhus trilobata) snowbrush ceanothus (Ceanothus velutinus) true mountain-mahogany willow Graminoids bluegrass (Poa spp.) brome (Bromus spp.) fescue (Festuca spp.) sedge (Carex spp.) wheatgrass (Agropyron spp., sensu lato) wildrye (Elymus spp.) Forbs alfalfa (Medicago spp.) aster (Asteraceae) balsamroot (Balsamorhiza spp.) beardtongue (Penstemon spp.) buckwheat (Eriogonum spp.) cinquefoil (Potentilla spp.) clover (Trifolium spp.) dandelion (Taraxacum spp.) fleabane (Erigeron spp.) lupine (Lupinus spp.) phlox (Phlox spp.) pussytoes (Antennaria spp.) sagebrush vetch (Vicia spp.) thistle (Cirsium spp.) yarrow (Achillea spp.)Forage preferences of mule deer vary among rangelands, seasons, and years, and appear strongly related to forage availability and plant phenology [41,215,327,348]. When green and succulent, forbs and grasses are selected over browse, but as forbs and grasses dry up, browse becomes increasingly important in the diet [41,215,236,241,327,348]. Leafless twigs are consumed only when other forage is scarce [348]. Severson and Medina [290] ranked mule deer food preferences in the Southwest from highest to lowest as follows: 1) fruits, flowers, and mushrooms; 2) new green herbage, particularly forbs and new leaves of deciduous shrubs; 3) new twigs and mature green herbaceous material; 4) new leaves and twigs of evergreen species, and 5) mature leaves and twigs of evergreen species. In general, mule deer forage is most abundant during the growing season and declines progressively in quantity, variety, and quality after annual growth ceases. On most mule deer rangelands, succulent forage is scarce in winter. However, in the mediterranean climatic region of California, succulent forage is abundant during spring, late fall, and winter and relatively scarce in summer. In some areas, mule deer forage is green and available year-round [88]. A review of mule deer diets in the Chihuahuan Desert reported that browse dominated diets in dry years and forbs dominated diets in wet years [340]. During a drought year in southeastern Arizona, mule deer and white-tailed deer diets changed from succulent deciduous forage to drought-tolerant evergreen species. The author suggested that competition with livestock may accentuate the effects of drought on mule deer and white-tailed deer diets [11]. See Livestock grazing for more information.
Deep snow makes forage less accessible to mule deer [348]. For example, on a treeless area of the Tillamook Burn, Crouch [87] estimated that 12 inches (30 cm) of snow would reduce available forage for black-tailed deer from about 224 kg/ha to 34 kg/ha [87]. Mule deer may paw through snow to feed, but they prefer to feed where there is no snow [119]. When other forage is buried by deep snow, conifer browse and arboreal lichens are important in mule deer diets in many regions [13,58,124,134,215,355].
Fire may affect mule deer diet composition. After a September prescribed fire on the east slope of the Colorado Front Range, mule deer diets in a montane shrub community contained more grass and less browse on burned plots compared with controls for 2 postfire years. In a montane grassland community, mule deer diets contained more grass and less browse on burned plots relative to controls during the 1st postfire year, but during the 2nd postfire year, there were no differences in grass and browse content [306]. For more information, see Indirect Fire Effects.
Nutrition: Protein content in preferred mule deer browse changes seasonally, reaching its lowest levels in winter. The time of year in which browse plants reach their highest nutritional level varies with the plant species [109,340]. Nutrients in mule deer forage species also change with seral stage [133]. Thus, diversity in browse composition and age is important for mule deer nutrition. For a review of the chemical composition of mule deer forage, see Kufeld and others [185].
Mule deer may select the relatively more nutritious foods from among those available (e.g., [223,325,327]). In interior Arizona chaparral near Globe, Arizona, combined mule deer and white-tailed deer use of sprouting shrubs during the first spring after a mid-September prescribed fire was positively related to moisture levels and crude protein content and negatively related to crude fiber content. For example, heavy use of true mountain-mahogany during the spring was associated with highest crude protein values and comparatively low crude fiber values, and selection of Wright silktassel (Garrya wrightii) during the summer growing season coincided with increases in moisture and crude protein and decreases in crude fiber [267]. However, in the Tillamook Burn in February—approximately 24 years after the 1951 fire in an area that had been salvaged logged—mule deer did not appear to select among 6 forage species (cascara (Rhamnus purshiana), red huckleberry (V. parvifolium), Douglas-fir, California hazelnut (Corylus cornuta subsp. californica), red alder (Alnus rubra), or vine maple (Acer circinatum)) based upon chemical composition, except that moisture tended to be higher in the most preferred species [262]. For more information, see Indirect Fire Effects.
Mule deer foraging effects: Because mule deer forage selectively, they can influence plant species composition and diversity by consuming palatable species, which may allow unpalatable species to gain dominance [80,135,315]. They can influence rates of nutrient cycling by altering litter quantity and quality and via urination and defecation. Also, mule deer may affect growth of stems and leaves and alter levels of plant nutrition [80]. Deer exert cascading effects on animals both by competing directly for resources with other herbivores and by indirectly modifying the composition and physical structure of habitats [3,80,264]. Reviews describing mule deer foraging effects are available: [24,80,220]. For information about mule deer effects on postfire succession, see Mule deer interactions with fuels and fire effects.
Fire has killed mule deer directly [68,117,125,147,196,291,335], but fire-caused mortality rates of large mammals are generally low (<1%) [117] and direct fire-caused mortality is thought to have little effect on large mammal populations [117,209]. The Greater Yellowstone Area fires of 1988 directly killed <0.1% of the approximately 2,500 mule deer summering within Yellowstone National Park [117,297]. After the 1939 Tillamook Fire, although there was "plenty of evidence" of black-tailed deer loss, black-tailed deer were "well represented everywhere" immediately after the fire (Einarsen 1939 cited in [68]). A 257,000-acre (104,000 ha) wildfire on the Boise National Forest, Idaho, killed "less than a dozen" deer and other wildlife species. A mule deer was directly killed by a 13-acre (5 ha) prescribed fire in chaparral in southern California [69]. Carcasses of 300 mule deer were found in a small area after the September 1928 Cahuilla Fire on the Stanislaus National Forest, California, burned in a horseshoe shape and trapped animals (Small 1928 cited in [291]). Another fire on the Stanislaus National Forest, the Anderson Valley Fire, killed 59 mule deer in only 212 acres (86 ha) [284]. Mule deer died in a Yolo County, California, fire that burned >12,000 acres (4,900 ha) in 1928 (Laing 1928 cited in [291]).
Large mammal mortality is most likely when fire fronts are wide and fast moving, fires are actively crowning, and thick ground smoke occurs [117,236]. Large fires may be more likely to result in death, injury, or eventual starvation of deer than small fires because large fires remove more protective cover and temporarily reduce forage (see Size and shape of burned areas) [284]. Nichols and Menke [236] noted that mule deer are mobile and generally able to flee chaparral fires, but large, rapidly moving wildfires may trap and kill them. A researcher observed some incidences of direct fire mortality of black-tailed deer following the 1939 Tillamook Fire in Oregon. The author also noted that although exact losses were not recorded for the 1933 Tillamook Fire, "it is reasonable to assume that destroying 84% of the cover in the 401-mile² (1,039 km²) area in a 20-hour period resulted in substantial wildlife losses" [147]. Necropsies revealed the primary cause of death of mule deer and other large mammals during the 1988 Greater Yellowstone Area fires was asphyxiation by smoke inhalation [117]. A mule deer in southern California chaparral apparently died of asphyxiation or heat during the 1957 Malibu area fire [69]. Shantz [291] noted many instances where the feet of deer were burned, thus crippling the animals.
As with other ungulates such as moose (Alces americanus) and elk, the number of fatalities caused by fire is likely related to season, population density, habitat type, terrain, fuel load, and prevailing winds [70,297]. Mule deer fawns may be most vulnerable to fire-caused mortality in spring during the hiding period (see Growth), when they are relatively immobile. Nichols and Menke [236] commented that mule deer fawns may be more susceptible to fire mortality because they cannot flee as quickly as adults. However, Collins [73] stated that young-of-the-year of most mammals, including deer, would have been able to escape an early-August mixed-severity wildfire on the Salmon National Forest, Idaho, in part because considerable escape terrain was available in the form of rock outcrops and slides. Sizer (1921 cited in [291]) noted that deer that hemmed against a rock bluff during the Mazatzal Fire on the Tonto National Forest in Arizona were able to escape the fire.
General observations suggest that mule deer use areas during and soon after fire (e.g., [107,117,196,291]). During an early August mixed-severity wildfire on the Salmon National Forest, deer were occasionally seen in areas still burning. For example, a doe and fawn were seen standing beneath a burning snag [73]. French and French [117] observed no large mammals fleeing a fire, and most appeared "indifferent" even to crowning fires.
Mule deer are most likely to disperse during the fawning period or during the rut [119]. One- to 2-year-old mule deer are most likely to disperse. Males are more likely to disperse than females [59,215,274,288,365]. In a migratory population in west-central Utah, few mule deer dispersed as fawns, but 60% of yearling males and 35% of yearling females apparently dispersed by 16 months old [274]. In the Missouri River Breaks, 70% of yearling males and 16% of yearling females dispersed [129].
Dispersal distances vary but are typically short. On Vancouver Island and in western Washington, male black-tailed deer dispersed 9.4 miles (15.2 km) and females dispersed 7.6 miles (12.2 km) on average. The maximum dispersal distances were 20 miles (32 km) for a male and 19 miles (30 km) for a female [59]. In Prairie County, Montana, migration distances ranged from 7 to 87 miles (11-140 km) for males and 8 to 16 miles (12-26 km) for females. Wood and others [365] suggested that long migration distances may be more characteristic of mule deer in patchy environments where suitable habitats are widely separated. The longest dispersal distance reported as of this writing (2012) was for a male mule deer in west-central Utah that dispersed at least 150 miles (240 km) [274].
Figure 2. Mule deer distribution and habitat in the western United States. Map courtesy of Remote Sensing and GIS Laboratory, Mule Deer of the Western United States. 2005. Utah State University, Logan, Utah. http://www.gis.usu.edu. Click on map for a larger image.
Mule deer are native to western North America. Scattered populations occur as far east as western Minnesota and Iowa. In Mexico, they occur south to Baja California (including some islands in the Sea of Cortez) and the southern end of the Mexican Plateau. They have been introduced in Hawaii and several islands in Prince William Sound [215]. Major gaps in mule deer distribution occur in the Mojave and Sonoran deserts in southeastern California, southern Nevada, southwestern Arizona, and northwestern Sonora, Mexico; the high-elevation or cold deserts and plains grasslands of northeastern Arizona and southeastern Utah; the Central Valley of California; and probably the Great Salt Lake desert region. Otherwise, mule deer occur in all of the biomes of western North America north of central Mexico, except the arctic tundra [7,345]. Within the mule deer's distribution, black-tailed deer occur along the northern Pacific Coast from central California north to southern Alaska [215].
States and provinces:
United States: AK, AZ, CA, CO, ID, KS, MT, NE, NV, NM, ND, OK, OR, SD, TX, UT, WA, WY
Canada: AB, BC, MB, NT, SK, YT [234]
Mexico [215]
Prescribed fire is commonly used in mule deer habitats. Both prescribed fire and wildfire can increase nutrient content and palatability of forage and make browse more abundant and accessible [127,235,328]. Fire may alter the level of nutrients in plants depending on season, soil, weather, fire type, and other factors [27,209]. A review stated that nutrient contents of plants after moderate- to high-intensity fire are generally higher than those of plants growing on unburned areas [290]. Short duration, low-severity fires may not result in an increase in plant nutrients [290,308]. Although increased plant nutrient levels after fire may last up to 20 years, most studies of moderate-severity or severe fires indicate that they revert to prefire or control levels in 2 years or less [27,100,209,290]. Burning may increase the palatability of mule deer forage by removing litter and stimulating new, succulent growth (e.g., [166]). Vegetation >5 feet (1.5 m) tall is inaccessible to mule deer (see Diet), and fire can increase mule deer forage accessibility by reducing browse height [170,194,290].
Because most of the mule deer's annual diet is browse, enhancement of browse is "key" to providing sufficient mule deer forage throughout the year [292]. While many forage species for mule deer increase after fire, others decrease. For example, 40 acres (16 ha) of mature quaking aspen-Engelmann spruce forest, Douglas-fir/mallow ninebark forest, and hawthorn (Crataegus spp.) shrubland were clearcut and burned in early spring in the Absaroka Range in south-central Montana. Saskatoon serviceberry and chokecherry were prominent understory shrubs. Two years after the fire, density of quaking aspen and willows had increased compared to prefire levels due to sprouting, but Saskatoon serviceberry and chokecherry densities were reduced [123].
Some researchers cautioned against using prescribed fire in oak habitats to avoid removing important mule deer forage and/or cover. Removal of mature, acorn-producing oak trees was often cited as detrimental to mule deer. Anderson (1969 cited in [66]) considered Gambel oak rangeland in Colorado to be important mule deer habitat. He cautioned against any treatment of Gambel oak as a general policy because of the importance of its acorns and browse to mule deer. Kruse [178] suggested using prescribed fire in Gambel oak woodlands on poor-quality sites to enhance brushy growth but avoiding prescribed fire use on better-quality sites with mature oaks. Taber and Dasmann [327] cautioned against using prescribed fire in California oak woodlands. They suggested that oaks be left untreated to provide acorns for black-tailed deer [327]. Guidelines for managing interior Arizona chaparral for mule deer included leaving Sonoran scrub oak (Quercus turbinella) untreated (USDA Forest Service 1970 cited in [345]). For more information, see Southwest shrublands.
Because fire may reduce important cover, several authors cautioned against using prescribed fire in mule deer habitats where cover is limiting. This is a management concern particularly on mule deer winter rangelands in desert grasslands, plains grasslands, prairie, southwestern shrubsteppe, pinyon-juniper woodlands, sagebrush, and desert shrub communities of the Great Plains, Southwest, and Great Basin regions [112,172]. Fairchild [112] suggested that particular attention be paid to maintaining cover in "key" wintering areas where mule deer concentrate. Suminski [318] cautioned against using prescribed fire and other treatments in areas containing sagebrush, antelope bitterbrush, and/or Stansbury cliffrose unless "excess" winter rangeland was present, because these species are important as winter forage and cover, and they are easily killed by fire. For more information, see Great Basin shrublands.
Most authors recommend creating a mosaic of burned and unburned habitats within a landscape to benefit mule deer (e.g., [57,146,292]). In a review of fire effects on ungulates in the northern Great Plains, Higgins and others [146] stated that "optimum" benefits of fire occur where fire creates a mosaic pattern of burned and unburned vegetation that provides new forage growth, seasonal habitats, and vegetation in early to late stages of succession. Nichols and Menke [236] stated that in general, optimal wildlife habitat is created in California chaparral when fire results in a mosaic of different age classes of shrubs interspersed with grassland. In high-elevation big sagebrush communities in Wyoming, Cook and others [79] recommended creating a mosaic of different-aged burned and unburned habitats for mule deer and other ungulates, burning a treatment unit once every 15 to 25 years. According to Stevens [311], diversity of food and cover over short distances is the key to enhancing mule deer populations in big sagebrush areas, with the distribution and pattern of shrub stands being more important than the quantity of shrubs [311]. Mule deer use pinyon-juniper woodlands in all stages of succession. Using prescribed fire or mechanical methods to create a mosaic of different successional stages in large areas of homogeneous pinyon-juniper woodlands may benefit mule deer by providing forage in early successional stands and mature forests nearby for cover (see Great Basin woodlands) [290]. Clark and Starkey [71] suggested that because mule deer forage heavily upon shrubs on most western winter rangelands and consume mostly herbaceous plants in spring and summer, prescribed fires to improve forage quality should provide a mosaic of burned and unburned areas. Prescribed fire may be less beneficial to mule deer in areas already having a mosaic of habitats. In an area with sparse herbaceous forage in the surrounding landscape in Lava Beds National Monument, average mule deer pellet group density 2 years after a prescribed fire was higher in burned areas than prior to the fire. However, in an area where herbaceous forage was abundant in the surrounding landscape, average mule deer pellet group density was similar before and 2 years after the fire [261].
Gruell [127] listed several factors that influence postfire plant composition, including the severity, size, and season of the fire, fuel type, prefire vegetation composition, and postfire foraging intensity (see Mule deer interactions with fuels and fire effects).
Fire timing: Prefire vegetation composition and season of burning may affect the availability of mule deer forage, particularly during the 1st postfire growing season. Several studies have compared effects of spring and fall burning on mule deer in California chaparral. In general, spring burning may be most beneficial to mule deer in this habitat because sprouts typically appear in 3 to 4 weeks, producing highly nutritious forage during both the dry summer months and the 1st postfire winter. Burning in spring also tends to favor crown-sprouting species such as chamise, a "staple" black-tailed deer food [170]. Biswell and others [34,36] cautioned that if a fire occurs in California chaparral after September, there may be little to no crown sprouting until the following spring, which would leave little to no cover or forage for overwintering black-tailed deer. Burning in fall may also favor shrubs that establish only by seed [320,327] and thus take longer to provide substantial mule deer forage. Fall fires and spring fires that occur prior to mid-March may stimulate germination from seeds as well as sprouting. Some important browse species reproduce from seed after fire, including wedgeleaf ceanothus, wavyleaf ceanothus (Ceanothus foliosus), and Stanford's manzanita (Arctostaphylos stanfordiana) [293]. Biswell and others [34,36] noted the importance of spring burning to black-tailed deer browse production but suggested use of fall burning in some areas to increase forage diversity. For more information, see California shrublands. In high-elevation big sagebrush communities in Wyoming, Cook and others [79] recommended burning in spring to minimize damage to shrubs and perennials and minimize first-year increases in weedy annual species. For more information, see the Fire Case Study on antelope bitterbrush. See also Great Basin shrublands.
Fire type: Very frequent fires in California chaparral may reduce important browse species [35,36]. A review of fire in California chaparral concluded that reburning 1 to 3 years after a fire may cause high mortality of sprouting plants such as chamise. Shrub mortality may increase when shrubs are heavily browsed by mule deer or livestock after reburning (see Mule deer interactions with fuels and fire effects) [38]. Reburning that occurs after plants have matured and produced seeds, which may be 15 years or longer, is most likely to maintain wedgeleaf ceanothus and other highly palatable, nonsprouting species in chaparral. Reburning before this point may reduce these species, and thus reduce carrying capacity for mule deer [35]. According to a review by Kinucan [170], the chaparral canopy reaches full development about 12 years after fire. Fires <9 years apart tend to kill seedlings, weaken sprouting response, and retard seed production [170]. Frequent fires in interior Arizona chaparral may reduce or eliminate some browse species for mule deer, including true mountain-mahogany, Wright silktassel, and hollyleaf buckthorn (Rhamnus ilicifolia) [259,290].
Patchy burns may be best for mule deer. After a wildfire in San Antonio Canyon, California, burned areas with "scant cover" were sparsely occupied by mule deer, while many mule deer (35% of observations) occurred in a burned area that had some cover remaining, even though it comprised only about 12% of the study area (Bartholomew 1942 personal communication cited in [291]). According to a review, patchy burns in mountain shrublands and chaparral rangelands have the greatest value to mule deer [153]. Other authors refer to the value of creating a mixture of early and late-successional habitats [43,54,146,209,239]. For more information, see Size and shape of burned areas.
Fire size: Regardless of habitat, small burns are often considered better for mule deer because portions of large burns may be left entirely unused by mule deer [9,27,41,93,236,291]. Miller [230] suggested that if burned areas are too large in chaparral habitats, most shrubs grow out of reach quickly, whereas small burned areas may provide browse for longer due to hedging by mule deer. Shantz [291] considered burning in small patches best for wildlife in general. The reduction of vegetation by fire over a large area may cause short-term food shortages for mule deer [236,284]. For example, large stand-replacing wildfires in chaparral habitats may reduce critical mule deer winter rangelands, which could lead to overgrazing and starvation during the first postfire winter [236]. Bendell [27] hypothesized that mule deer may benefit most from small fires because they result in more edge and greater interspersion of habitats than one large fire. However, Gates (1968 cited in [27]) noted that black-tailed deer in 2 areas on the east coast of Vancouver Island had similar population densities despite contrasting amounts of edge and interspersion resulting from wildfires and patchy clearcut logging over large areas. Although a large fire could potentially reduce the interspersion of food and cover for mule deer by producing uniform vegetation, reviews stated that fires rarely burn evenly and typically result in a mosaic of vegetation beneficial for deer [27,209].
Small burns provide some advantages over large burns, but a disadvantage is that heavy deer browsing may reduce or eliminate preferred sprouting trees and shrubs from small burns [291]. For example, several authors noted that small burned areas or clearcuts in quaking aspen forests may concentrate mule deer and other browsing animals to the point where quaking aspen browse is eliminated (see Mule deer interactions with fuels and fire effects) [248,290]. For this reason, Brown [56] suggested burning multiple small areas within a landscape to disperse animals. Alternatively, a single, large fire that creates a mosaic of vegetation may create favorable mule deer habitat while still dispersing animals [56]. In bluebunch wheatgrass grasslands in the Rocky Mountains, concentrations of animals on small burned areas may increase interspecific competition by increasing diet overlap between mule deer, elk, and bighorn sheep [162,306].
Other considerations: Because travel patterns of mule deer prior to fire may affect postfire use, Carpenter and Wallmo [66] suggested it is important that Habitat management efforts for mule deer be based on the particular movement patterns and needs of the individuals making up that population. Dasmann [92] suggested that because black-tailed deer may not use a burned area outside of their home range, numerous small burns spaced within about 0.5 mile (0.8 km) of one another will benefit more black-tailed deer than a few large, widely spaced burns (see Fire size). He also suggested that because black-tailed deer use different aspects at different times of day and year (see Topography), prescribed fires should be spaced to include a variety of aspects, including southern and northern exposure, and slope positions, including ridgetops and canyon bottoms [92]. In California chaparral, black-tailed deer consume different forage species at different times of year. During summer, species growing on cool, north-facing slopes and streambeds are used. In late fall, black-tailed deer move to warm, south-facing slopes where they consume herbaceous plants and chamise [170]. Thus, prescribed fires in cold stream bottoms and on north-facing slopes are unlikely to benefit black-tailed deer on winter rangelands, whereas prescribed fires on south-facing slopes may be beneficial [327]. Urness (1974 cited in [345]) recommended treating interior Arizona chaparral on various slopes and aspects due to the changing seasonal requirements of mule deer, while maintaining sufficient cover for security.
Prescribed burning and its associated human activities may reduce mule deer populations in the short term by increasing their vulnerability to hunting. Two years after an October mixed-severity prescribed fire in Gambel oak shrublands in western Colorado, mule deer pellet group densities during fall, winter, and spring were 25% less than before the fire. This was due, in part, to heavy hunting pressure and lack of cover after the fire [184]. Mule deer in Gambel oak rangelands in western Colorado may not have benefited from prescribed burning because of heavy hunting pressure [181]. In coastal Oregon, declining black-tailed deer populations protected from hunting increased following fire. The author cautioned that fire and logging may increase mule deer herds only if they are protected from hunting [110]. Sampson [284] cautioned that hunting restrictions may be needed to maintain populations in burned areas due to greater hunter success.
Proximity of habitats to water may affect their use after fire, particularly in the Southwest. Biswell and others [36] suggested that mule deer might not respond positively to postfire habitat improvements in areas where water is lacking. See Water management for more information.
The presence of cattle and other livestock may reduce the benefits of prescribed fire to mule deer. Tiller and others [273,332] suggested that because cattle and mule deer compete for space within chamise chaparral burned under prescription on the San Bernardino National Forest, California, cattle should be excluded during the 1st and 2nd postfire years. See Livestock presence in burned areas for more information.
Fire affects the spread of nonnative invasive plants, which may be beneficial or detrimental to mule deer. For more information on mule deer use of nonnative invasive plants, see Nonnative invasive plants. See also FEIS reviews of nonnative invasive plant species of interest.
Mule deer may affect postfire succession. For more information, see Mule deer interactions with fuels and fire effects.
Fire may influence interspecific interactions. For example, mule deer may avoid postfire successional communities if elk are present (see Mule deer, other ungulate, and fire interactions). Asherin [15] suggested using several small prescribed fires scattered across winter range in order to reduce interspecific interactions and disperse browsing pressure across burned and adjacent unburned areas.
In California chaparral, fuel breaks may provide travel ways, increase interspersion of herbaceous vegetation, and increase edge that benefits mule deer [93,145,291].
For a 2002 analysis of the economic costs and benefits of implementing a prescribed burning program in southern California for increasing mule deer and other big game habitat, see Loomis and others [202].
Historically, mule deer occurred in most habitats of the western continental United States except some desert ecosystems of the Southwest (see General Distribution). Thus, they were probably adapted to a wide range of FIRE REGIMES. Mule deer occur in habitats with historically short (e.g., bluebunch wheatgrass grasslands) to long (e.g., Sitka spruce-western hemlock forests) fire-return intervals, and in areas with understory FIRE REGIMES (e.g., redwood and ponderosa pine/grassland), mixed-severity FIRE REGIMES (e.g., riparian shrublands and ponderosa pine-Douglas-fir forests), and stand-replacement FIRE REGIMES (e.g., Wyoming sagebrush steppe and mountain hemlock forests). The Fire Regime Table summarizes characteristics of FIRE REGIMES for vegetation communities in which mule deer may occur. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find FIRE REGIMES".
Fire exclusion beginning in the early 1900s resulted in increased tree density in formerly open rangelands in much of the Southwest and Great Basin. This may have increased habitat for mule deer from what occurred historically (see Threats). Clark and Starkey [71] cautioned that although rangeland ecosystems evolved with fire, they are not in their "natural" condition today because of a legacy of fire exclusion, livestock grazing, agricultural development, introduction of nonnative invasive plants, construction of roads and other barriers, and other factors.
Adult mule deer establish and traditionally use seasonal or year-round home ranges. According to reviews, mean annual home range sizes for mule deer vary from 74 to 34,220 acres (30-13,850 ha) [7,135,215]. Nonmigratory black-tailed deer have among the smallest home ranges [324].
According to a review, mule deer in "open, simple, and more variable habitats" tend to have larger home ranges than those in "closed, diverse, and stable environments" [215]. For example, in open, nonforested northern prairie habitat in Prairie County, Montana, year-long home ranges of migratory and nonmigratory adult mule deer ranged from 452 to 16,306 acres (183-6,599 ha) [365], whereas in forested prairie breaks habitat, year-long home ranges of migratory and nonmigratory adult mule deer were smaller, ranging from 114 to 8,253 acres (46-3,340 ha) [129]. According to Wood and others [365], seasonal home ranges tend to be small among mule deer in mesic mountainous habitats and large in more xeric, desert habitats. In the northern Great Plains in South Dakota, mule deer occupying rough terrain had smaller home ranges than those in relatively level terrain [288]. In Prairie County, Montana, nonmigratory mule deer had small home ranges and relatively high local population densities in areas with interspersed hardwood draws and badlands. Mule deer ranged over larger areas and occurred at lower densities in areas where badlands and hardwood draws were lacking or more widely separated [365]. In arid mixed woodland-grassland communities in north-central New Mexico, larger home ranges tended to have a greater proportion of grasslands (r²=0.18, P=0.037) [29].
Males tend to have larger home ranges than females [7,135,215]. In the Bridger Mountains of Montana, winter home ranges averaged 613 acres (248 ha) for males and 603 acres (244 ha) for females. Summer home ranges averaged 593 acres (240 ha) for males and 437 acres (177 ha) for females [243]. In the Missouri River Breaks region, the presence of fawns-at-side was the most important factor in determining home range sizes of adult females, with adult females with fawns-at-side having smaller home ranges than nonreproductive females. Home range sizes of adult females were not related to population density, forage condition, or age of females [129].
Seasonal movements and daily activity patterns may be strongly influenced by air temperature, wind, and/or snow depths [92,215]. In Colorado, mule deer tended to move within their winter habitats to areas with temperatures of 16 to 45 °F (-9 to 7 °C). When temperatures were below 16 °F, they tended to increase their metabolic rate (Mautz and others 1985 cited in [215]). During cold weather mule deer tend to use south-facing slopes, and during warm weather they tend to use north-facing slopes (see Topography) [92]. High winds appear to influence mule deer movements only during periods of cold weather, when mule deer seek shelter and reduce their activities [92,215]. According to Mackie and others [215], snow depth probably has the most influence on mule deer movements. Deep snow makes forage less accessible, increases energy expenditure, and may increase an animal's vulnerability to predation [78,135,237,348]. Snow depths of about 12 inches (30 cm) generally impede mule deer movement, especially that of young animals, and may cause mule deer to move to areas with less snow [78,135,204,215].
In regions with snow, winter ranges are often smaller than summer ranges. In the Bridger Mountains of Montana, average snow depths and patterns restricted mule deer to <20% of their total year-round range in winter. Under severe snow conditions only 20% to 50% of the winter range was considered usable [243]. Gilbert and others [122] concluded that because snow was too deep (>18 inches (46 cm)) in Middle Park, Colorado, during 2 of 3 winters, >90% of mule deer winter range was uninhabitable. In cold and snowy periods on Vancouver Island, mule deer used <40% of the area used during mild winters (McNay and Doyle 1987 cited in [58]).
In the arid Southwest, summer ranges may be smaller than winter ranges because of limited water. In Maricopa County, Arizona, mule deer had smaller home ranges in summer than winter, possibly due to greater dependence on limited water sources during periods of hot weather [115]. The 400,000-acre (162,000 ha) summer range of the Kaibab mule deer herd in Arizona was smaller than its winter range [156].
Fidelity to traditional home ranges can be so great that deer will "starve to death" rather than travel "a few kilometers" to abundant forage (Dasmann and Taber 1956 cited in [135]). During a fire, mule deer may not leave their home ranges even as their home ranges burn. If they do leave, they typically return soon after fire. Shantz [291] noted that mule deer and white-tailed deer returned to their home ranges so soon after fire that they burned their feet. Humphrey (1926 personal communication cited in [291]) noted that mule deer on the Manti-La Sal National Forest, Utah, returned to their burned home ranges "even though the forage was practically all destroyed". For more information, see Travel patterns. Although mule deer are unlikely to explore unfamiliar but favorable areas even if these areas are only a few kilometers away, they may shift their use of an area over time to exploit changes in resource availability [58]. Livestock grazing may affect the use of mule deer home ranges. For more information, see Livestock grazing.
Several small fires may be more beneficial to mule deer than one large fire because of increased edge habitat. A review of published and unpublished reports by students and faculty at the University of Nevada stated that while mule deer occurred 0.25 mile (0.40 km) into a large (4-mile² (10 km²)) burned area on winter range in the Jack's Valley Habitat management Area near Carson City, use of antelope bitterbrush was concentrated at the edge of the burn, within only about 450 feet (140 m) inside and outside of the burned area [173]. Because mule deer often prefer to feed close to cover, they may not use interiors of large burned areas [236]. Immediately following large, stand-replacing fires in California chaparral, mule deer grazed no farther than 300 feet (90 m) from cover [14].
Large fires may be detrimental to mule deer in the short term by removing cover [36]. Dubreuil [104] suggested that selection for unburned habitat by mule deer 1 year after a 83,500-acre (334,800 ha), mixed-severity August to September wildfire in the southern Black Hills was related to the relative lack of cover and forage in burned areas. In the North Coast Ranges of California, mule deer densities were higher during the first 5 postfire years in "opened brushland" consisting of scattered islands of shrubs and herb-dominated openings than in a large burned area with few shrub islands. The authors suggested that the lack of cover in the large burned area kept mule deer densities lower [36]. For more information, see Fire size.
The elimination of vegetation by fire over a large area may result in initial food shortages for mule deer. Wildfire that denudes low chaparral areas, often critical to mule deer populations for winter forage, can lead to overgrazing and starvation [236]. The shortage of food after a chaparral fire is usually short-lived, however, because sprouting often begins within a few days after fire and provides excellent forage. For more information, see California shrublands.
Fire may reduce the numbers of external and internal parasites that affect mule deer and other animals [27,146,209], although the effect is likely brief. Fires can reduce winter tick (Dermacentor albipictus) populations [144]. After a May prescribed fire in mature quaking aspen forest and willow habitat in Elk Island National Park, Alberta, the number of engorged adult female ticks and larvae immediately declined. Winter tick survival was highest where the burn was patchy and duff consumption was least [103]. According to a review, the number of winter ticks killed also depends on the habitat type and season of burning because most ticks are found on the tops of shrubs in spring [146]. Although winter tick populations may be reduced in the short term, fire's long-term effects on winter tick populations were unknown as of this writing (2012).
Fire in wetland habitats may help reduce giant liver fluke (Fascioloides magna) populations, which may be detrimental to mule deer and white-tailed deer [327]. Giant liver flukes have a complex life cycle that involves an intermediate aquatic snail host for the embryonic stage, aquatic vegetation for the larval-cyst stage, and an ungulate host for the juvenile and adult stages [331]. In east-central Alberta, deer, elk, moose, and American bison (Bos bison) populations were heavily infected with giant liver flukes (Swales 1936 cited in [331]). In order to control these infestations, dead aquatic vegetation was burned and aquatic snails were controlled with chemicals. This "apparently eradicated" giant live flukes in ungulates in the area; limited examinations found no giant liver flukes in deer harvested by hunters (Stock 1978, Pybus 1990b, cited in [331]). Following the Tillamook fires in Oregon, black-tailed deer were free of liver flukes (Fascioliasis) and lungworms (Strongylida) that had been prevalent before the fires. Apparently, fire had killed the dryland snail that is the intermediate host for liver flukes and some lungworms [160,369].
Mule deer affect vegetation development and plant productivity after fire. Total herb production on deer and elk rangeland in Arizona increased 3-fold on both understory and stand-replacement burned plots compared with unburned control plots after mixed-severity wildfire in a ponderosa pine forest. Total herb production remained higher in understory-burned plots than in control plots for at least 9 years after fire. However, it was higher in stand-replacement burned plots than in control stands for only 2 years after fire, possibly because of heavy grazing by livestock and wildlife [242]. On Quail Ridge, California, prescribed fire in blue oak woodlands resulted in blue oak and interior live oak sprouting. However, heavy postfire browsing by mule deer reduced sprout production: 95% of oak sprouts were grazed by mule deer [12]. In west-central Montana, Saskatoon serviceberry showed considerable damage from deer browsing following a severe wildfire in a Douglas-fir forest [82]. Postfire mule deer and elk browsing pressure influences the response of quaking aspen stands to fire (e.g., [118,165,203,232]). In the Grand Canyon, Fule and others [118] concluded that high mule deer herbivory reduced quaking aspen regeneration for 60 years, leading to white fir- and Douglas-fir-dominated forests with fuels able to support stand-replacing fire. The authors hypothesized that had excessive mule deer herbivory not occurred, the fuel structures of quaking aspen-ponderosa pine forests would probably be less susceptible to crown fire and more conducive to long-term ponderosa pine survival. They noted, however, that white fir may still have come to dominate the stand in the long-term absence of fire [118].
Fire severity may influence the effect of mule deer browsing on postfire plant growth. On the Hastings Reservation in Monterey County, California, browsing was heavier on postfire growth of plants that had all of their stems consumed by fire than on postfire growth of plants where old, charred stems remained. Chamise plants with old stems remaining had stems with significantly longer root crown sprouts over an 8-year period following an August wildfire (P<0.001) [95].
Mule deer in most cases do not alter the successional trajectory of large burned areas, but the course of succession may be altered in small burned areas that receive heavy browsing pressure, particularly if other early seral habitats in the landscape are sparse [232,248,290]. For example, Mueggler and Bartos [232] noted that mule deer browsing prevented quaking aspen regeneration in small clearcuts and in an uncut quaking aspen forest on summer rangeland in southern Utah, but quaking aspen in nearby large burned areas regenerated successfully. They suggested that burned areas or clearcuts <5 acres (2 ha) would concentrate mule deer use, reducing quaking aspen regeneration [232]. In Colorado, logging of several large (15-20 acres (6-8 ha)) areas at one time resulted in successful quaking aspen regeneration despite large mule deer and elk populations (Crouch 1983 cited in [24]). See Lotan and others [203] for suggestions regarding size of burned areas and burning intervals for deer in quaking aspen stands.
Prior to fire, mule deer selection of preferred forage may alter the abundance and kinds of fuels [209]. By removing fine fuels, mule deer may reduce the likelihood of surface fires. They may also enhance the development of unpalatable trees that may act as ladder fuels [364]. Wisdom and others [364] made the following generalizations regarding the interactions between fires and herbivory by mule deer, elk, and cattle in forested landscapes in western North America:According to a review, deer in boreal forests, including mule deer, are usually associated with early successional stages of burns. Fire stimulates rapid growth of deciduous shrubs, which increases the food supply for deer. As trees regenerate and their crowns close, the food supply is reduced, resulting in lower deer populations [282]. Stand-replacing fire in boreal forests can greatly increase the production of woody browse for moose [210] and likely for mule deer.
Prefire stand age and species composition play an important role in plant response to fire in boreal forests [210,213]. The benefits of burning to moose, and possibly mule deer, may peak 20 to 25 years after stand-replacing fire and last less than 50 years [210]. See the FEIS review of moose for more information on fire effects on moose browse in boreal forests. Although mule deer and moose may consume many of the same browse species in this region, such browse is likely to grow out of reach of mule deer and thus become inaccessible to mule deer before becoming inaccessible to moose, which are taller [45]. In addition, herbaceous plants in postfire successional communities tend to be more important to mule deer than to moose.
Mule deer browse species may be more nutritious in early than late succession [282]. Stand-replacing fire in boreal forest often increases the protein, phosphorus, calcium, magnesium, and potassium content of woody browse for up to 3 postfire growing seasons [210].
California
California shrublandsAccording to a review, burning effects on chaparral vegetation depend on time of year. The quality of annual growth on unburned shrubs in chaparral is highest (15%-20% crude protein, depending on species) during rapid growth in spring and declines during the dry season when plants are dormant (4%-15% crude protein, depending upon species). If an area is burned in August or September, there is usually some sprouting in November or earlier, but most growth begins in April or May. Sprout growth continues during the early summer, when sprouts are high in protein (20%-30%) and moisture. Protein levels decline thereafter but remain higher than those in unburned chaparral vegetation until the 2nd postfire summer. If the fire occurs in spring, sprouting typically occurs within a few weeks after the fire. Protein levels following spring burning follow the same seasonal trend as those on fall-burned areas but are typically much higher than that of the fall-burned areas. This may be due to ash deposition and resultant abundant nitrogen after spring burning. In fall-burned areas, the ash has been leached away before it is used in spring. Unless growth of shrubs is controlled by moderate browsing or other means, many sprouting shrubs become unpalatable or unavailable to black-tailed deer within a few postfire years [170].
Lake County studies: A series of related studies reported nonmigratory black-tailed deer densities in chamise and mixed chaparral following fire in the North Coast Ranges of Lake County, California (e.g., [34,36,37,90,320,322,323,326,327]). These studies examined black-tailed deer use of 3 habitats: 1) mature, dense chaparral, with nearly pure chamise stands on south-facing slopes and mixed chaparral stands with interior live oak on north-facing slopes (hereafter, mature chaparral); 2) a large area of chamise and mixed chaparral burned in a September 1948 wildfire with scarce unburned islands (hereafter, burned chaparral); and 3) treated chaparral habitat with an "opened brushland" of interspersed patches of dense shrubs and herbs (hereafter, treated chaparral). The latter area was burned in a series of small prescribed fires, then seeded with native and nonnative herbs. Some of the area was burned again by a wildfire in August 1948 [36,90,326]. Burned and treated chaparral had higher quantity and quality of black-tailed deer forage than mature chaparral. Because of herbs seeded on the treated chaparral site, the average yearly proportion of grasses and forbs in the diets of black-tailed deer 1 year after fire was highest there (45%). It was higher in burned (14%) than mature chaparral (10%). Protein content in annual black-tailed deer diets averaged 17% on burned and 14% on treated chaparral 1 year after the fires, but only 9% on mature chaparral. All 3 habitats had a similar seasonal pattern, with black-tailed deer diets highest in protein in spring and lowest in fall after shrubs had ceased growing and grasses and forbs were mainly dry. The authors suggested that black-tailed deer diets in burned and treated chaparral had higher protein content than mature chaparral because of the higher proportion of high-protein, herbaceous forage in winter and early spring in these habitats. Shrubs in these habitats were shorter, and leaves, which were higher in protein than stems, were more available [327].
Black-tailed deer densities in burned and treated chaparral peaked during the1st postfire summer, then declined. In the burned chaparral, black-tailed deer were sparse in September immediately after fire because of lack of food. Density increased in burned chaparral in October and November after shrubs had sprouted [326]. It peaked at 120 black-tailed deer/mile² the 1st postfire year as black-tailed deer emigrated from surrounding areas and increased productivity [90,326]. Fawn production and survival increased in the burned chaparral during the 1st postfire spring and summer, probably due to increased herbaceous forage and accessibility of shrubs [90]. Production was higher in burned chaparral (1.15 fawns/doe) than in nearby mature chaparral (0.7 fawn/doe) the 2nd postfire spring [14,321,322]. However, the population declined the 2nd postfire summer because reduced forage quantity and nutritional quality led to many animals dying of malnutrition during July and August [90,326]. The population decreased in subsequent years (Table 3) [90,321,326]. Trends in black-tailed deer densities in treated chaparral were similar to those in burned chaparral. The July following treatments, black-tailed deer densities peaked at 131/mile² then declined to 82/mile² the 5th postfire summer (Table 3) [326]. Overall, black-tailed deer densities were higher in treated than burned chaparral because seeding of herbs in treated chaparral led to higher forage production [323]. In addition, higher black-tailed deer density in the treated chaparral kept sprouts hedged and accessible longer [36]. Bissell [32,34] noted that although densities in mature chaparral were lower than those in burned and treated chaparral (about 30 black-tailed deer/mile² [326]), mature chaparral provided more acorns, an important fall food. This suggests the importance of habitat heterogeneity to black-tailed deer in chaparral habitats.
Table 3. Black-tailed deer population density in burned and treated chaparral in Lake County, California [326] Summers since fire Black-tailed deer/mile² Burned chaparralTreated chaparral
1* 120 131 2 106 112 3 52 103 4 44 85 5 no data 82 *Black-tailed deer density in nearby, mature chaparral during this summer was 30/mile².Black-tailed deer body weight may improve following fire. During the hunting season in Lake County, California, bucks from treated and burned chaparral tended to be heavier than bucks in mature chaparral. Doe weights tended to be highest in treated chaparral, intermediate in burned chaparral, and lowest in mature chaparral. Researchers suggested that black-tailed deer body weight was higher in treated and burned chaparral because of higher-quality summer diets compared with mature chaparral [34,36,327].
In contrast to studies in Lake County in the North Coast Ranges, a study in the Central Coast Ranges found no increase in black-tailed deer population size after prescribed fire in chaparral. Instead, the density of black-tailed deer was significantly higher in oak woodlands and grasslands than in burned chaparral for all seasons (P<0.05 for all comparisons), except the 2nd growing season after fire. Density in chaparral did not increase until the 2nd growing season after fire, and then declined to prefire numbers within 6 months. Density increased again during the 3rd postfire growing season, but the increase was not as great as the previous growing season. No significant change in fawn survival occurred after the fire, and the increase in density during the growing season was attributed to female groups moving into chaparral from oak woodlands rather than to an increase in population size. The results demonstrated that burning chaparral may not result in increased black-tailed deer numbers. The lack of an effect of burning was attributed to habitat heterogeneity and the juxtaposition of chaparral habitats near oak woodlands and grasslands in this Central Coast Range site [174].
Other California shrublands: Dasmann [94] stated that fire offers no short-term benefits to mule deer on winter rangelands on the east side of the Sierra Nevada because it removes important winter browse and cover. However, in the Owens Valley, California, a study reviewed the effects of 3 wildfires and 2 prescribed fires on mule deer winter rangelands dominated by blackbrush (Coleogyne ramosissima) and concluded that fire increased plant species diversity and mule deer carrying capacity on most blackbrush rangelands during the first "few" years after fire by increasing annuals, shrub seedlings, and sprouts [26].
California woodlands: Black-tailed deer pellet group counts in spring were 1.7 times higher on burned sites than adjacent unburned sites after a prescribed fire near Trinity Reservoir, northern California. The vegetation type was a gray pine-Oregon white oak-California black oak (Quercus garryana-Quercus kelloggii)/wedgeleaf ceanothus-true mountain-mahogany woodland. Black-tailed deer appeared to be attracted to the burned area due to a flush of new herbaceous growth in early spring. By postfire year 4, however, pellet group counts were similar among burned and unburned areas [167]. For more information about black-tailed deer use of oak habitats after fire, see California shrublands.
California forests: In California forests, mule deer may benefit from availability of both burned and unburned areas. On the western slope of the Sierra Nevada—in areas within a giant sequoia (Sequoiadendron giganteum) grove that were treated by cutting, piling, and prescribed burning—mule deer forage was more abundant and utilized more heavily by mule deer on treated than untreated, control areas. In addition, many mule deer forage species (e.g., California hazelnut, ceanothus, canyon live oak, Sierra mountain misery (Chamaebatia foliolosa), and sedge) were higher in protein on treated than untreated areas. The results were attributed in part to increased sunlight on treated areas. The authors concluded that prescribed burning favored the germination of seeds of the shrubs most valuable for mule deer. However, the optimal mineral balance resulted from mule deer feeding on both treated and untreated sites because some mule deer forage species, such as Pacific dogwood (Cornus nuttallii), had higher calcium and phosphorus levels on shady, control sites than on sunny, burned sites [191].
The benefits of logging and prescribed fire to black-tailed deer may be short-lived. According to a review, black-tailed deer density in redwood forests of northern California increases following fire and logging from about 3/mile² to >70/mile², with the greatest increase occurring from about 5 to 10 years after disturbance. After 10 years, shrub and tree cover becomes dense or grows out of reach and protein content declines. Black-tailed deer then decrease in number until 10 years after disturbance, when they reach their original low levels [91].
Great Basin
Great Basin shrublands: Fire may have short-term negative effects on mule deer in the Great Basin and other parts of the Intermountain West by reducing cover and important winter forage species such as sagebrush, antelope bitterbrush, desert bitterbrush (Purshia glandulosa), and Stansbury cliffrose [126,142,146]. Klebenow [172] noted that mule deer avoided large burned areas on a Nevada winter range until antelope bitterbrush and other shrubs recovered, which often took 15 years. The carrying capacity of California's Lassen-Washoe winter rangelands was reduced after large wildfires in the 1980s increased mule deer susceptibility to winter mortality because of loss of shrub cover. Small openings in sagebrush communities may favor mule deer by increasing habitat heterogeneity, but large fires that reduce heterogeneity and cover are typically detrimental [339]. However, lack of fire for long periods in Great Basin shrublands may reduce shrubs as succession proceeds to pinyon and juniper [146], thus reducing important winter forage.Antelope bitterbrush and sagebrush, particularly big sagebrush, are important mule deer browse species in the Great Basin and elsewhere that may be reduced by fire. Antelope bitterbrush's response to fire is variable, depending upon genetics and site characteristics, but it is likely to decrease following even low-severity fire. In south-central Oregon, antelope bitterbrush declined following low-severity fire and showed little increase in cover during the subsequent 5 to 6 years [61]. In southwestern Montana, 8 years after a prescribed fire in an antelope bitterbrush-mountain big sagebrush-bluebunch wheatgrass community, antelope bitterbrush density did not differ between burned and unburned sites, but antelope bitterbrush cover, flower production, and seed production were less on burned than unburned sites. Mountain big sagebrush density and cover were less on burned sites, while total herbaceous cover was similar on all sites [116].
Frequent fire could reduce the amount of browse available to mule deer by eliminating antelope bitterbrush from large areas. In an antelope bitterbrush-cheatgrass community in south-central Washington, vegetation production, cover, and species composition were similar on unburned areas and areas burned in wildfires in 1963 and again in 1970, except that antelope bitterbrush and big sagebrush were killed by burning and had not established in the 8 years following the 1970 fire [272].
A summer wildfire in Wasatch County, Utah, killed most antelope bitterbrush plants, and postfire growth was slower for plants within the burned area. The number of mule deer pellet groups was higher in unburned areas (16-36 groups/100 feet²) than burned areas (4-8 groups/100 feet²) 4 years after the fire. Burned areas also had fewer big sagebrush plants than unburned areas, so the difference in mule deer use between burned and unburned areas appeared to be due to reduced forage availability and probably deeper snow on burned areas [142]. In south-central Oregon, antelope bitterbrush declined following low-severity fire and showed little increase in cover during the subsequent 5 to 6 years. The authors suggested that burning in a mosaic pattern may enhance antelope bitterbrush regeneration, and thus mule deer habitat, by maintaining scattered, unburned plants as seed sources [61].
Klebenow [172] provided the following generalizations about mule deer use of antelope bitterbrush communities after fire: Initially, mule deer tend to avoid large burned areas, particularly in winter, although they may be attracted to some burned areas in early spring when green vegetation first becomes available. From 10 to 15 years after fire, mule deer may again use the burned area if cover of antelope bitterbrush and other shrubs ranges from about 10% to 15%. The timing of antelope bitterbrush reestablishment varies, depending in part on the distribution of seed caches by rodents, weather, and cattle use of the burned area [172]. The effects of fire on antelope bitterbrush, and thus mule deer, depend in part on fire severity and timing. Severe late summer fires in Idaho killed 66% of the antelope bitterbrush, while a moderate-severity spring fire in Montana killed only 33%. A summer fire of moderate severity in Oregon killed an entire stand of antelope bitterbrush [127]. See the FEIS review of antelope bitterbrush for more information.
Some researchers reported increases in herb and/or shrub productivity and nutritional quality after fires on sagebrush rangelands [302,336]. In south-central Wyoming , a study investigating effects of 2 wildfires and 3 prescribed fires on production of herbs and shrubs in mesic, high-elevation big sagebrush communities found production of perennial herbs on burned sites averaged twice that on unburned controls by postfire year 3, while production of annual herbs varied little 2 to 3 years after fire. Productivity of important browse species increased and generally compensated for mortality, which was <15% for Saskatoon serviceberry, 55% for antelope bitterbrush, and 25% for true mountain-mahogany. Crude protein content of herbs from late spring through early fall was significantly higher on burns for 2 years after fire (P≤0.05). The authors speculated that the consistent increases in plant productivity and nutrient concentrations across all burned sites resulted from the mesic conditions [79]. A review by Nielson and Hinckley [238] stated that mule deer prefer rangelands containing a variety of herbs and shrubs to those dominated by big sagebrush, noting that when more palatable shrubs are available in winter, mule deer use of big sagebrush decreases.
A review stated that on mule deer sagebrush-grassland winter rangelands "it is possible to have short-term and long-term benefits (from fire), but there is a great possibility of both short-term and long-term losses as well". The author described effects of fire in antelope bitterbrush and sagebrush communities as follows [172]:
Short-term effects (<15 years):The effect of prescribed fire in pinyon-juniper woodlands on mule deer depends on what plant species were present in the understory prior to fire and whether deep snow occurs in the area [41,111,192,225,290]. Everett [111] cautioned that prefire composition in pinyon-juniper stands will most likely determine the postfire plant composition. If sprouting shrubs are present before fire, they will likely come back following fire. If sprouting shrubs are not present, perennial grasses are likely to develop [41]. In pinyon-juniper woodlands adjacent to interior Arizona chaparral stands with similar shrub composition, prescribed fire may result in increased production of sprouting shrubs and increased herbaceous growth. In the Coconino and Kaibab plateaus in Arizona, however, most understory shrubs (for example, Stansbury cliffrose and big sagebrush) associated with pinyon-juniper woodlands are easily killed by prescribed fire. However, herbage production may increase [290]. On the Hualapai Indian Reservation in Arizona, herbage production increased significantly after prescribed and wildfires in pinyon-juniper communities. As a result, mule deer used burned areas more than unburned areas during most of fall and winter (P<0.10). Stands burned in prescribed fires ranged from 4 to 12 years old, and a stand burned in a wildfire was 15 years old. Because snow was not deep in this study area, shrubs were not critical winter forage items. In addition, precipitous terrain provided cover for mule deer. After 13 to 15 years, browse plants were more abundant in unburned areas, but grasses and forbs were more abundant on burned areas [225].
In pinyon-juniper woodlands, mule deer often concentrate their activities near edges of burned areas. In eastern Nevada and eastern California, an evaluation of 11 wildfire burns in pinyon-juniper communities ranging from 2 to 115 years old found that mule deer pellet groups and tracks did not occur inside recently burned areas in a grass-forb stage of succession; instead, pellet groups were concentrated within 330 feet (100 m) of the burned edge in the unburned pinyon-juniper woodland. On burned areas in a shrub-dominated stage of succession, more deer pellet groups were found within the burned area away from the edge than within 160 feet (50 m) of the edge. Big sagebrush and steep and broken topography substituted for tree cover in these areas [307]. In pinyon-juniper woodland on the Hualapai Indian Reservation, mule deer used the edges of burned areas more frequently than they used the interiors of burned areas or unburned areas. Pellet group accumulation rates were highest in burned areas 1,320 to 2,640 feet (400-800 m) from unburned areas [225].
See Other treatments for more information on mule deer use of pinyon-juniper woodlands.
Great Basin forests:
Ponderosa pine: Fire in ponderosa pine stands may benefit mule deer by increasing understory vegetation quantity and nutritional quality [33,289,290]. According to a review, nutrient responses in ponderosa pine in Arizona vary with the type of understory, age and structure of the forest, and season. However, prescribed fire generally increases nutrient availability and concentrations, which improves forage quality for mule deer and other wild ungulates for at least the 1st postfire growing season [289]. A study in Arizona ponderosa pine found that in the 1st growing season after fire, crude protein, phosphorus, and in vitro digestible dry matter were higher in mule deer forage from areas burned in a severe May wildfire than in adjacent unburned controls. Increases in phosphorus and digestible dry matter lasted to the 2nd postfire year but increases in protein did not. By the end of the 2nd growing season, however, there were no differences in nutritional content of mule deer forage between burned areas and unburned controls [249].
Mule deer use of ponderosa pine stands may increase after burning in response to increased forage production and edge, although responses vary in the first few postfire years. Near Flagstaff, Arizona, deer use of a ponderosa pine forest that had been burned in a high-severity May wildfire increased for the first 2 years after the fire, then became "inconsistent" during the 3rd year, possibly due to reinstated cattle grazing on the burned areas [177]. In another ponderosa pine forest in Arizona, mule deer use of burned areas decreased in all seasons the 1st postfire year. Summer and fall use then increased 2.5 times more than use on an unburned control through the rest of the 20-year study. In winter and spring, however, mule deer use returned to control levels for a few postfire years, then increased to 120 times more than that of the control at the end of the study. Increased use was likely the result of increased amount of edge and forage production, especially forbs and ceanothus, on the burned areas [205]. In a recently logged ponderosa pine forest on the Coconino National Forest that burned in a May wildfire, deer pellet densities were higher in a moderate-severity burned area during postfire summers 1 to 3 than in an unburned control. However, pellet group densities were higher in the control than in a high-severity burned area during postfire summers 1 and 2. During postfire summer 3, pellet group densities were higher in the high-severity burned area than the control (Table 5). The result was attributed to the production of palatable herbaceous species on burned areas. Herbaceous plant production was similar on all sites during the 1st postfire summer (range: 452-582 pounds/acre). During the 3rd postfire summer, production averaged 1,651 pounds/acre on the high-severity burned area, 1,275 pounds/acre on the moderate-severity burned area, and only 559 pounds/acre on the unburned control [64].
Table 5. Mean deer pellet groups/acre in a logged and burned ponderosa pine forest on the Coconino National Forest, Arizona, 1 to 3 summers after wildfire [64] Summers since fire Moderate-severity fire High-severity fire Unburned control 1 1,001 257 672 2 398 191 267 3 363 262 116Although total biomass of grasses and forbs often increases in ponderosa pine forest after fire, the quantity of useable mule deer forage may actually be less on burned areas if species composition shifts to relatively unpalatable species [210]. In the short term, prescribed understory burning failed to improve herbaceous forage production for deer in ponderosa pine stands near Flagstaff, Arizona. Although herbaceous plant production increased dramatically, nonnative common mullein (Verbascum thapsus), an unpalatable species, dominated the understory 1 year after fire [113].
Quaking aspen: Quaking aspen communities are important summer and fall rangelands for migratory mule deer throughout the West [203,248,290]. Leckenby and others [193] rated quaking aspen communities in the Great Basin shrubsteppe ecosystem as 2nd only to riparian zones in value to mule deer. According to a review, quaking aspen is among the top 10 preferred browse species for mule deer, and understory species in quaking aspen communities provide abundant forage [203]. Another review stated that prescribed fire in quaking aspen parklands may benefit mule deer and white-tailed deer by: 1) top-killing sprouting woody plants; 2) providing a seedbed for establishment of forage species; and 3) increasing the nutrient level and digestibility of browse and herbs the first 2 years after burning [19]. The effects of prescribed fire on quaking aspen stands and fire's resulting effect on mule deer depend, in part, upon the amount of postfire quaking aspen sprouting. Young quaking aspen trees are more likely to sprout than old trees [290]. See the FEIS review of quaking aspen for more detailed information. Maximum sprout densities are typically realized the 1st and 2nd postfire years, followed by a gradual decline [290]. Abundant mule deer browse is typically available for 5 to 8 years following burning, at which time leafy crowns typically grow beyond the reach of mule deer. Small burned areas or clearcuts may attract concentrations of mule deer and other browsing animals, to the point where quaking aspen browse is eliminated (see Mule deer interactions with fuels and fire effects) [248,290]. Thinning quaking aspen stands, rather than burning or clearcutting, may promote herbaceous understory production rather than quaking aspen sprouting [290]. Mature quaking aspen stands may provide better cover for mule deer than clearcut stands [333]. See these reviews on managing quaking aspen for mule deer and other wildlife: [248,333].
Great Plains
Great Plains grasslands: Historically, mule deer in the Great Plains were largely confined to breaks and rough terrain, where shrubs were protected from fire [146]. According to a review, tree and shrub cover in the Great Plains is limiting to mule deer populations, so fire that removes available cover may have a negative effect on mule deer. However, fire that results in sprouting of trees and shrubs may benefit mule deer by increasing browse, and after such plants are tall enough, cover [290]. In parts of the northern Great Plains where snow is deep, optimum mule deer habitat may not be reached until 30 or more years after fire, when woody plants used as cover have developed on grassland sites. However, the absence of fire from grasslands for >50 years may result in tree encroachment, canopy closure, and reduction of herbs and shrubs, thus reducing mule deer habitat quality [146].In the Great Plains, grassland fire may result in early green-up of warm-season grasses, improved seed germination, and increased production of grasses and forbs [210], which may be beneficial to mule deer on summer rangelands. Many studies that reported increased production of forage after fire also described some circumstances under which production was reduced. In general, fires followed by drought and fires in areas averaging <11 inches (300 mm) of summer rainfall may result in decreased forage production for mule deer and other ungulates [210]. On plains rough fescue (Festuca hallii) grasslands, prescribed fire may reduce litter, making plains rough fescue more palatable to mule deer. If burning is done in a "wet" year, prescribed fire may maintain a stable forage supply, but if done in a "dry" year, prescribed fire can reduce forage production for 1 to 4 years [19]. See the FEIS review of rough fescue for more information.
Great Plains woodlands and forests: In the Great Plains, hardwood and shrubby draws, cottonwood (Populus spp.) floodplains, and north-slope Rocky Mountain juniper communities are important mule deer habitats [287]. According to a review, fires in draws and floodplains may result in sprouting of trees and shrubs important to mule deer as food and cover (e.g., chokecherry, silver buffaloberry, green ash, boxelder, and bur oak (Quercus macrocarpa)). Small fires in Rocky Mountain juniper communities likely benefit mule deer by increasing the number of openings, improving shrub growth, and increasing plant diversity [287]. In the Missouri River Breaks region, Eichhorn and Watts [108] measured plant succession over 10 years following wildfires that occurred 1 to 28 years prior to the start of the study. In Douglas-fir-Rocky Mountain juniper stands, grass cover was significantly reduced during the first 3 postfire years compared to unburned controls (P<0.05) but was greater than in unburned controls during postfire years 8 to 28. Forb cover peaked during postfire year 3 and then decreased, but it remained above unburned controls in all years (P<0.05). Shrubs "steadily" increased after burning. Total shrub cover exceeded that on unburned controls during postfire years 5 to 28 (P<0.05). Cover of sprouting shrubs such as chokecherry, rose, and snowberry, however, exceeded that on unburned controls after the 1st postfire year. Rocky Mountain juniper was "eliminated" on all burned areas during postfire years 1 to 28. The study sites had "little or no" livestock grazing pressure [108].
In the Missouri River Breaks region, mule deer likely benefit from increased grasses and forbs on burned areas as long as some cover is retained. Prescribed fires conducted over 2 weeks from late May to early June resulted in a 1,100-acre (445 ha) mosaic of unburned, lightly burned, and severely burned areas. Mule deer thermal cover did not differ among unburned, lightly burned, and severely burned areas. However, hiding cover was significantly less on lightly burned (32%) and severely burned areas (21%) than on unburned areas (45%) 1 year after the fire (P<0.05 for all variables). Grass cover did not differ among burned areas during the 1st and 2nd postfire summers. Forb cover was greater in lightly and severely burned areas during the 1st postfire year. During the 2nd postfire year, forb cover was similar in unburned, lightly burned, and severely burned areas in May and June, but in July and August, forb cover in burned areas exceeded that of the unburned control. Browse cover decreased to <1% in lightly burned and severely burned areas during the 1st and 2nd postfire summers, while unburned areas had significantly more browse. Mule deer pellet group counts indicated the mule deer used all treatments similarly during the 1st and 2nd postfire years, and habitats were not selected or avoided based on cover or forage availability [366].
Pacific Northwest
Pacific Northwest grasslands: Fire in grasslands may increase palatability and accessibility of some grasses. In big sagebrush/bluebunch wheatgrass (Pseudoroegneria spicata) and Douglas-fir/bluebunch wheatgrass communities of interior British Columbia, the proportion of available bluebunch wheatgrass used by tame mule deer in April was highest the spring after a November prescribed fire. It was lowest in unburned control areas. However, total use of bluebunch wheatgrass by mule deer was less on burned areas than unburned areas because availability was less. The authors suggested that mule deer foraged preferentially on bluebunch wheatgrass in the burn despite its relatively low abundance there because of its improved palatability and accessibility (due to removal of litter) [362].
Pacific Northwest forests: Agree [1] described black-tailed deer response to postfire succession in western hemlock-Douglas-fir forests of the Pacific Northwest. In the 1st year after fire, shrubs sprout and conifer seedlings are abundant. Black-tailed deer populations generally increase on the burned area, particularly on the perimeter. Twenty years later, the understory is dense and saplings are heavily browsed; black-tailed deer populations continue to expand. As the canopy closes, the lack of sunlight in the understory reduces shrub and herb cover and black-tailed deer populations decline. After 200 to 250 years, the forest begins to resemble old growth, with small openings caused by disease and windthrow [1]; at this time, black-tailed deer populations presumably increase. Cowan [81] reported that the carrying capacity of black-tailed deer in coastal conifer forests on Vancouver Island increased gradually to a peak in approximately 10 to 15 years after fire. However, data provided by Bendell [27] showed that black-tailed deer populations peaked during postfire years 1 and 2 and then declined the subsequent 13 years. His study was based upon hunter success following a 31,000-acre (12,500 ha) August wildfire in the Sayward Forest on the east coast of Vancouver Island. No data were available regarding prefire hunter success or hunter success in control areas [27].
Figure 5. Mule deer harvested per hunter during the 13 years following a large wildfire on Vancouver Island. No data were collected during postfire year 8 [27].Population density immediately following the 1939 Tillamook Fire was <1 black-tailed deer/mile² [110]. At this time, <5% of the area within the burn perimeter supported green trees [147]. During postfire years 1 to 3, when black-tailed deer were protected from hunting, populations increased to >15 black-tailed deer/mile². Most of the population increase was attributed to an increased number of births and decreased mortality, but some was also likely due to immigration from adjacent areas of poorer forage. Protein in black-tailed deer browse (e.g., vine maple, salmonberry, thimbleberry, red alder, red huckleberry, and red elderberry (Sambucus racemosa)) on the Tillamook Burn increased after the 1939 fire and then declined. For example, protein content in vine maple was 12.8% 3 years after the fire and 9.3% 6 years after the fire [109]. A comparison of adult black-tailed deer body weights in 1943, 4 years following the 1939 fire, and again in 1966, 27 years following the 1939 fire, showed that as the seral vegetation developed in the burned area and black-tailed deer populations increased, black-tailed deer body weights declined [147].
Postlogging practices employed in mule deer habitats in the Pacific Northwest often include prescribed fire, and black-tailed deer populations may be higher on logged and burned sites than on untreated sites. On the western Olympic Peninsula, Washington, black-tailed deer and elk used 2 clearcuts in western hemlock forest that were burned in a "patchy" prescribed fire in late May. Some plots were also fertilized, seeded with grasses and forbs, and planted with western hemlock, western redcedar, and Douglas-fir seedlings. It took 2 years for the treatments to be completed. During postfire year 2, mule deer and elk pellet group densities were higher on unburned plots than burned plots. During postfire years 3 and 4, pellet group densities were higher on burned than unburned plots, coincident with peak forage grass production in burned areas. During postfire year 5, when grass production declined markedly, pellet group densities were higher on unburned than burned plots [195]. On the Clemons Tree Farm in coastal Washington, black-tailed deer densities peaked in midsuccessional Douglas-fir-western hemlock forests after logging and prescribed fire. High densities in midsuccession were thought to occur because of use of these forests for cover (Table 2) [55]. In contrast, at the Starkey Experimental Forest and Range in northeastern Oregon, within a 30-mile² (78 km²) area enclosed by a fence, fuels in western spruce budworm (Choristoneura occidentalis)-killed grand fir-Douglas-fir forests were reduced by thinning and broadcast burning or slash pile burning in September or October. Between 1 and 5 years after treatment, patterns of stand use indicated no effects of fuels reduction treatments on ≥2-year-old does; mule deer either avoided treated areas or used all stand types proportional to their availability across seasons (spring and summer) and scales within the 19,300-acre (7,800 ha) landscape. The authors suggested that control stands likely provided better foraging opportunities than treated stands, particularly during hot summer months, because of rapid senescence of understory vegetation in areas with open canopies [200].
Table 2. Black-tailed deer density in 4 seral stages of Douglas-fir-western hemlock forests on the Clemons Tree Farm in coastal Washington [55] Black-tailed deer/mile² Seral stage Description 35 soon after disturbance* recently logged and burned forest that was "just starting to produce deer forage" 34 early succession 10 to 25 years after logging and burning when the food supply peaked 57 midsuccession* logged and burned forests in midsuccession "when the forests had reached, or passed, their peak productivity" 36 late succession* dense, mature 2nd-growth or old-growth forests with low forage productivity *Time since disturbance not specified.Postlogging site preparation practices may benefit mule deer in the short term by increasing forage quality. As mentioned earlier, protein in black-tailed deer browse generally increased after the 1939 Tillamook Fire, then declined [109]. However, forage quantity and/or quality may not always increase on burned areas. Throughout western Washington, there was little difference in January protein content of black-tailed deer foods (e.g., trailing blackberry, salal (Gaultheria shallon), western redcedar, blue huckleberry (Gaylussacia frondosa), and red huckleberry) from 4 seral stages of Douglas-fir-western hemlock forests. The seral stages ranged from recently logged and burned forests to mature forests. The author stated that he may not have detected a difference because of high variability among samples and small sample sizes [55].
Increased forage abundance on logged and burned sites may increase black-tailed deer body condition. In coastal Douglas-fir, western hemlock, and spruce forests of western Oregon, harvested black-tailed deer bucks on recently burned forest averaged 213 pounds (97 kg), whereas harvested bucks in unburned, closed-canopy forest averaged only 125 pounds (57 kg). The protein content of black-tailed deer's most preferred browse (e.g., salal, red alder, salmonberry, thimbleberry, and red huckleberry) was higher on the burned forest than the unburned forest and may have contributed to the greater weight of black-tailed deer in logged and burned forest [109].
Rocky Mountains
In contrast, fire in Rocky Mountain shrublands may be detrimental to mule deer by removing important browse. For example, a Wyoming big sagebrush (Artemisia tridentata subsp. wyomingensis)/bluebunch wheatgrass winter rangeland on the East Fork of the Salmon River in Idaho was burned under prescription in fall. Grass production decreased slightly the 1st year after burning but returned to prefire levels 2 years afterwards. Frequency of bluebunch wheatgrass was reduced for 2 years but returned to prefire levels by the 3rd postfire year. However, Wyoming big sagebrush, a preferred mule deer browse species, was removed from the site for at least 4 years after burning [252].
Rocky Mountain forests: Lyon [207] provided a generalized description of mule deer and white-tailed deer response to postfire succession in forests in the northern Immediately following a severe fire, the landscape may appear barren and provide little forage for deer. As early as the 1st growing season after fire, some woody seedlings may appear, and plants not killed by fire may sprout. Within the first few postfire years, forbs and grasses dominate the area, and shrub cover increases. As shrub cover increases, forbs and grasses decrease. If the shrubs are palatable to deer, they can provide abundant deer forage. Shrub dominance may continue from 10 to 100 years after fire, but shrubs are eventually displaced by trees. In mature forests, understory vegetation is typically sparse and provides little forage for deer [207]. Plant succession on large, severely burned areas may be slow compared with that on small burns because of low plant survival within burned areas and remoteness of seed sources [207,235]. A review stated that the positive effects of fire on deer forage generally last <30 years [210]. An "intense" prescribed fire in Douglas-fir forest in Idaho improved forage for mule deer and elk; the improvement was expected to last >20 years [208].
Mule deer may benefit from the development of shrubfields resulting from fire in forests of the Rocky Mountains and elsewhere (e.g., [15,16,222]). On a mule deer ponderosa pine-Douglas-fir winter rangeland in the Rattlesnake River drainage near Missoula, Montana, a wildfire in 1919 resulted in shrubfields dominated by snowbrush ceanothus and mallow ninebark (Physocarpus malvaceus). About 40 years after the fire, the shrubfields were developing into forests, and mule deer populations appeared to be declining as a result [171]. Large fires in Idaho during 1910, 1919, and 1934 created extensive shrubfields in mule deer winter range. In the 1950s, a helicopter survey of the North Fork Clearwater River drainage found that 68% of winter rangeland for mule deer burned between 1889 and 1949, with 41% of the area burned in 1919. In the Selway River drainage, fires burned 32% of the mule deer winter rangeland (Norberg and Trout 1957 cited in [370]). The postfire successional shrubfields appeared to favor mule deer [370]. Mule deer use of postfire successional shrubfields may decrease as vegetation grows tall and out of reach. In the Lochsa River area, Idaho, prescribed fires in spring and fall decreased shrub height to a level accessible to mule deer and elk. Four years after burning, 3 primary mule deer and elk browse species (Rocky Mountain maple (Acer glabrum), willow, and Saskatoon serviceberry) averaged nearly 10.5 feet (3.2 m) tall, with about 80% of their twig production within reach of the ungulates [194].
Most studies of fire effects in Rocky Mountain forests indicate that burning favors mule deer by increasing forage. A review reported that grass and forb biomass generally increases the first 5 to 10 years following stand-replacing fires in Rocky Mountain forests [210]. Quaking aspen stands are important mule deer foraging habitats in the Rocky Mountains and throughout the West (see Great Basin forests) [203,248,290]. Grass and forb biomass decreased the 1st growing season after fire in quaking aspen stands in western Wyoming but increased the 2nd and 3rd growing seasons to above prefire levels. On severely burned sites, grass recovered more slowly than forbs [23]. Mule deer primarily foraged in burned habitats 3 winters after an August mixed-severity wildfire. The fire burned 2,700 acres (1,100 ha) of mule and white-tailed deer winter rangeland in the Selway-Bitterroot Wilderness, Idaho. Mule deer used burned Douglas-fir/mallow ninebark and ponderosa pine/bluebunch wheatgrass communities more frequently than their availability during winter. In spring, mule deer still preferred burned Douglas-fir/mallow ninebark and ponderosa pine/bluebunch wheatgrass communities but increased use of other habitats, including burned grand fir/white clintonia (Clintonia umbellulata) communities. Shrubs that sprouted after fire, particularly Saskatoon serviceberry, mallow ninebark, and Scouler willow (Salix scouleriana), were most heavily used by mule deer [166].
Burning may increase the palatability of mule deer forage in Rocky Mountain forests. In the Selway-Bitterroot Wilderness, Idaho, mule deer used mallow ninebark more on burned areas than on unburned areas. The authors suggested that mallow ninebark palatability was increased by burning [166].
Snags may provide important cover for mule deer in burned areas. In a conifer forest near Laramie and Saratoga, Wyoming, mule deer pellet groups were 1.3 to 9.3 times greater on plots burned in wildfires than on nearby, similarly-aged clearcut plots 5 and 10 years after disturbance. This was attributed in part to a greater variety of forage species and to greater hiding cover provided by snags in burned areas (633-722 snags/ha) compared to clearcuts (0-66 snags/ha) [96]. Eight years after a severe August wildfire in a lodgepole pine forest near Fort Collins, Colorado, mule deer used the burned area more than adjacent, unburned lodgepole pine stands from September to June (P=0.01), apparently because of abundant cover provided by snags and abundant seeded grasses in the burned area [279].
Although fire may create snags that provide cover, fire may also remove important cover from Rocky Mountain forests. Deer pellet group counts were "negligible" during the winter and spring immediately following the Moose Creek Fire on the Salmon National Forest, and they were substantially reduced from prefire counts during postfire year 1. Prefire cover within and adjacent to the burned area was limited due to previous logging and the natural sparseness of the forest. The fire removed much of the remaining cover, and only one "sizeable" patch of cover remained. The author noted that despite road closures, hunting pressure on deer using the burn during the fall immediately after the fire was high. The fire was a mixed-severity August wildfire in a mosaic of curlleaf mountain-mahogany/bluebunch wheatgrass, bluebunch wheatgrass/needle-and-thread grass, spiny grease bush (Glossopetalon spinescens), mountain big sagebrush, ponderosa pine, and Douglas-fir communities [73]. Lack of cover and forage in burned areas may limit mule deer use. One year after a 83,500-acre (334,800 ha), mixed-severity August to September wildfire in the southern Black Hills of west-central South Dakota and northeastern Wyoming, all radio-collared mule deer selected ponderosa pine/true mountain-mahogany/Rocky Mountain juniper habitat with >70% cover and a grass-forb and shrub understory for foraging and bedding. Meadow habitats were avoided. Burned ponderosa pine habitat was used in proportion to availability. During summer, radio-collared mule deer selected unburned ponderosa pine habitat and avoided burned ponderosa pine, unburned ponderosa pine/quaking aspen, and meadow habitats [104].
Postlogging site preparation in mule deer habitats in the Rocky Mountains may include prescribed fire, and mule deer browse production may be higher on clearcut and burned sites than on unburned clearcut sites. In Mineral County, Montana, mule deer browse production was higher 5 to 6 years after clearcutting and burning under prescription (48.4 pounds/acre) than after clearcutting alone (20.8 pounds/acre). The result was attributed to increased production of redstem ceanothus (Ceanothus sanguineus) and evergreen ceanothus (C. arboreus), both of which had abundant sprouting on clearcut and burned areas [356].
Postfire seeding of conifers may reduce the amount of time that abundant mule deer forage is available. In western Montana, mule deer browse biomass 37 years after fire was lowest within Douglas-fir/mallow ninebark habitat that was seeded with Douglas-fir soon after fire. It was highest in unseeded postfire successional shrublands (Table 6) [356].
Table 6. Mule deer forage biomass (pounds/acre) on 2 sites in western Montana, 37 to 39 years after wildfire [356] Location Years of fire Years since last fire Stand characteristics Forage biomass Boyd Mountain 1910, 1931 37 dense tree canopy as a result of seeding with conifers after fire 1 1910 37 no forest overstory and dense shrubs 91 Tamarack Creek 1929 39 no forest overstory and dense shrubs, particularly evergreen ceanothus 496Nutritional quality of mule deer forage species in the Rocky Mountains may increase, decrease, or remain unchanged by burning. Any effects are usually short-lived. One year after prescribed fire in western redcedar/Oregon boxwood (Paxistima myrsinites) communities on mule deer winter range in northern Idaho, 4 browse species (redstem ceanothus, willow, serviceberry, and Rocky Mountain maple combined) were higher in moisture and crude protein content on burned sites than unburned sites. However, the effect was absent during postfire years 2 and 3 [15]. In western larch-Douglas-fir stands in Montana that had been burned with "light, moderate, or hot" understory fires 3 years previously, nutrient content of plants was compared with samples from stands not burned for 70 years. Sodium levels were higher for several mule deer forage species (heartleaf arnica (Arnica cordifolia), fireweed (Chamerion angustifolium), and white spirea (Spiraea betulifolia)) in stands where at least half the duff was consumed by fire (i.e., moderate or severe fires). Iron levels were significantly greater in some forage species on burned than unburned sites (heartleaf arnica, dwarf bilberry (Vaccinium caespitosum)). Calcium (white spirea, fireweed) and phosphorus (white spirea) levels were significantly lower on burned than unburned sites (P≤0.05 for all variables). Of the species tested, none that occurred in both burned and unburned sites (e.g., heartleaf arnica, dwarf bilberry, white spirea, and fireweed) showed significant differences in nitrogen, manganese, or copper content due to burning [309]. During the 4 years following a late-summer wildfire in a xeric ponderosa pine forest and adjacent montane grasslands used as winter-spring rangeland by mule deer in the Selway-Bitterroot Wilderness in Idaho, mineral concentration in herbaceous plants tended to be similar between burned and unburned sites. However, nitrogen and potassium were lower on burned sites than unburned controls the first year following the fire [229].
Better nutrition on burns may lead to increased body condition of mule deer. After a 83,500-acre (334,800 ha), mixed-severity August to September wildfire in the Black Hills of west-central South Dakota, increased mule deer body condition during the 2nd and 3rd postfire years was attributed to increased nutritional quality of forage in burned areas [353].
Fire may shift plant composition in communities, which may harm or benefit mule deer. In western larch-Douglas-fir stands in Montana, unburned control sites were covered by palatable mule deer forage species such as white spirea, huckleberry (Vaccinium spp.), twinflower (Linnaea borealis), and grasses, whereas sites burned in a "hot" understory prescribed fire were largely covered by Marchantia polymorpha—a liverwort with unknown palatability to mule deer—and fireweed, which is a highly palatable and nutritious mule deer forage species. Species composition of sites burned with a "light" understory fire was similar to that of unburned control sites [309]. Mule deer populations may not increase after a fire if the fire does not increase palatable forage. Over 5,400 miles² (14,100 km²) burned in the 1988 Greater Yellowstone Area fires, including 42% of Yellowstone National Park [49,296]. Mule deer populations declined 19% during the first winter after the fires. Singer and others [297] speculated that mule deer may have declined because they were consuming less palatable shrubs after the fires.
Southwest
Southwest grasslands: Fires may improve palatability of plants in southwestern grasslands. Fires that burn off the spines from cacti (cholla (Cylindropuntia spp.), pricklypear (Opuntia spp.), and barrel cactus (Ferocactus spp.)) make cacti more palatable and/or available as forage for mule deer [210,221]. In grazed southwestern shrubsteppe south of Tucson, Arizona, deer were attracted "almost immediately" to an area that was burned under prescription in November, in part because of the attractiveness of pricklypear. Nearly all pricklypears from which spines were burned were consumed by deer and other animals "within a few weeks" [221]. In desert grasslands, old growth of tobosa (Pleuraphis mutica), big sacaton (Sporobolus wrightii), and Johnson grass (Sorghum halepense) is relatively coarse and unpalatable to mule deer, white-tailed deer, and other ungulates, but their new postfire growth is succulent and readily eaten [341].Fires at the grassland-woodland ecotone may remove woody vegetation without increasing ground cover [210], which may be detrimental to mule deer. For more information, see FEIS reviews of species of interest.
Southwest shrublands
Interior Arizona chaparral: Mule deer are common in interior Arizona chaparral [143,290,319,345]. Because most shrub dominants sprout after fire or germinate quickly from seeds after fire, fire in this community may increase forage for mule deer [62,143]. Forbs and grasses develop rapidly after fire and are generally abundant for 3 or 4 years, followed by an abrupt drop to prefire levels in 2 to 3 more years, with forbs declining more rapidly than grasses. The decrease in herbs is associated with an increase in shrubs over time. Shrubs generally recover rapidly and dominate the site in about 5 years, regaining prefire values approximately 11 years after fire [290,345]. In the Mingus Mountain area, forb production peaked at about 281 pounds/acre in the 3rd postfire growing season after an 18,000-acre (7,300 ha) June wildfire, while grasses peaked at 213 pounds/acre in the 5th postfire growing season. Shrub cover and biomass were still increasing 6 years after the fire, when the study ended [246]. In the Three Bar Wildlife Area, forb and grass production was about 217 to 325 pounds/acre in the 5th and 6th postfire growing seasons and 109 to 110 pounds/acre in the 7th and 10th postfire growing seasons [143]. Longevity of increases in mule deer forage quantity in interior Arizona chaparral varies with productivity of the site. In interior chaparral in east-central Arizona that was seeded with nonnative weeping lovegrass (Eragrostis curvula) following a severe prescribed fire, shrub growth in the burned area was the most rapid the first 2 years following fire; by postfire year 5, shrub density was equal to that on the unburned control [62].
Burning may increase the nutrient content of mule deer browse in interior Arizona chaparral. Protein content of deer browse in recently burned areas in 3 regions of Arizona was generally higher than that in unburned areas but declined over time. Protein contents of plants on 9-month-old and 3-year-old burned sites were similar to those on adjacent, unburned sites, indicating that the effects of burning on plant nutritive quality were short-lived. Browse use by deer was much greater on burned than unburned sites [319].
Gambel oak: Mule deer consume Gambel oak leaves, twigs, and acorns [9,254]. For example, along the Wasatch Front in Utah, Gambel oak may provide up to 75% of the available winter browse [254]. Although important to wintering mule deer in terms of forage availability and palatability, Gambel oak ranks low in nutritional value [254]. It sprouts after fire, and fire in Gambel oak communities may result in abundant, succulent browse for mule deer. On the Uinta National Forest, Utah, examination of Gambel oak stands that had been burned 3 and 15 years prior to the study indicated that burned stands recovered to unburned control levels in 6 to 35 years, with stands at low elevations recovering faster than stands at high elevations (r=0.99, P<0.01). Mule deer wintered at low elevations, where postfire recovery of Gambel oak was most rapid [186].
Fire in Gambel oak communities may not always result in abundant browse. Two, 5, and 10 years after a fall mixed-severity prescribed fire in Gambel oak rangeland in western Colorado, total vegetation production decreased 17%, 27%, and 19%, respectively, compared to prefire levels. Most of the reduction occurred among shrubs, particularly big sagebrush and common snowberry. They decreased 38% during the 2nd and 5th postfire years and 46% after 10 years compared with prefire levels, partially as a result of dry weather. Relative to prefire levels, seeded and native grasses decreased by 5% after 2 years and 29% after 5 years, but they increased by 76% after 10 years. Forbs increased by 14%, 2%, and 17% after 2, 5, and 10 and postfire years, respectively [181].
Mule deer in Gambel oak rangelands in western Colorado may not have responded to prescribed fire and other treatments because of heavy hunting pressure [181]. They occurred at similar densities in treated (see treatments in Table 4) and untreated sites within Gambel oak rangelands 2, 5, and 10 years after treatment, except that densities were reduced 61% on chained sites 2 years after treatment. Burned sites had patches of unburned or partially burned vegetation, which increased mule deer access to forage and provided escape cover and shade. In sprayed areas, dead material remained standing for the first few years, leaving a thicket only slightly more open and accessible than before spraying. During posttreatment years 5 and 10, accessibility decreased further as dead materials fell against shrubs. Mule deer use of chained stands may have been reduced because chaining treatments reduced cover the most. During posttreatment year 10, chained areas were still open and accessible but cover had increased. The authors noted that hunting pressure on mule deer was very heavy during posttreatment year 2 [181].
Table 4. Mule deer use of prescribed burned, herbicide sprayed, or chained Gambel oak rangelands in western Colorado 2, 5, and 10 years after treatments [181] Years since treatment Control Treatment Prescribed fire* Herbicide spraying Chaining Mule deer density**Mule deer density
% change mule deer density*** Mule deer density % change mule deer density Mule deer density % change mule deer density 2 19.1 16.7 -13 15.0 -21 7.4 -61**** 5 17.6 24.2 +38 25.9 +47 24.1 +37 10 18.1 15.6 -14 26.0 +44 14.1 -22 *An October mixed-severity fire. **Number of mule deer/2.59 km². Based on mule deer pellet group counts and the average deer defecation rate of 13 groups/day. ***Calculated as the percent difference between treatments and controls. ****Denotes significance at P≤0.10.Fire may increase nutrition of plants in Gambel oak communities. Two growing seasons after a fall mixed-severity prescribed fire in Gambel oak rangeland in Colorado, zinc and copper levels were higher in forbs, grasses, and shrubs on burned than unburned sites. However, no differences were found in the protein, lignin, calcium, or phosphorus content of these plants on burned and unburned sites [181]. Less than 1 year after a 1,270-acre (514 ha) August wildfire burned Gambel oak and mountain big sagebrush on southwest-facing slopes in north-central Utah, nutrient values (protein, phosphorus, and in vitro digestibility) of Gambel oak buds and stems from burned stands were significantly higher than those of unburned stands (P<0.001). However, tannin content of the sprouts was also higher than that in unburned stands, and overall forage value of Gambel oak to wintering mule deer was relatively low [254]. In southwestern mixed-conifer forest on the Lincoln National Forest, New Mexico, the nutritional quality of wavyleaf oak (Quercus × pauciloba), Gambel oak, and alligator juniper—all important mule deer browse—was generally higher on burned sites 2 to 4 years after a severe April wildfire than on unburned sites. However, the authors cautioned that differences were unlikely to be biologically significant to mule deer. Nutritional quality of browse varied with the plant species' responses to fire, plant parts sampled (leaves or stems), and the season sampled (winter, fall, or spring) [228].
According to a review, whether mule deer use a Gambel oak community after fire depends on the structure of the community and type of vegetation present on adjacent areas as well as the type of fire and size of the burned area. Mule deer may benefit most from mixed-severity fires that create openings in very dense stands and increase herbaceous plant production in the understory [254]. In north-central Utah, mule deer use of Gambel oak browse on burned and unburned sites was similar. The authors suggested that mule deer may have used the sites similarly despite increased nutritional quality of browse on burned sites because cover on burned sites was reduced, important browse species such as mountain big sagebrush and antelope bitterbrush were reduced, and tannin content of forage was increased on burned sites. They suggested that burning may temporarily improve forage nutritional quality on Gambel oak rangelands but that, without repeated fire, the postfire proliferation of Gambel oak sprouts may ultimately result in dense, less usable Gambel oak forage and fewer understory species. Moreover, mule deer habitat quality may be reduced by the reduction of "fire-susceptible" browse species such as mountain big sagebrush and antelope bitterbrush [254].
Too frequent fire in Gambel oak communities may be deleterious to mule deer by reducing acorn crops because few acorns are produced from small diameter stems. See the FEIS review of Gambel oak.
Mule deer may be year-long residents in foothills, occupy seasonal ranges that include footslopes in winter and adjacent mountain slopes in summer and fall, or migrate between distinct, widely separated winter-spring and summer-fall ranges. Mule deer may move from high-elevation montane ranges in summer to low-elevation ranges in fall and winter [215]. In mountainous regions of the West, most mule deer are migratory, spending summer in the mountains and fall in foothills and valleys. Some mule deer live at low elevations year-round and in the absence of snow, most migratory mule deer remain at high-elevation summer ranges during winter [66,159,348]. Generally when snow is deep, mule deer move down from high elevations; move up from areas of low relief to slopes of southern or western exposure or to windswept ridges; or move into forests where snow is shallow. In regions with snow, fall migration usually depends upon the timing of snowfall and the depth of snow on summer and transitional ranges, whereas timing of spring migration is associated with snowmelt, the appearance of succulent forage on transitional and summer ranges, and perhaps the timing of parturition [7,135,215]. In the arid Southwest, mule deer may migrate in response to rainfall patterns [7].
Not all mule deer in a population migrate. Only 7% of female mule deer migrated in the Rocky Mountain foothills of eastern Colorado [183]. In the Poudre Canyon area of the Roosevelt National Forest, about 80% of mule deer were migratory [204]. In the Green River Basin of western Wyoming, 95% of mule deer were migratory [285]. In populations of north-central Colorado, the average proportion of mule deer that migrated was 52% but ranged from 0% to 100% [75].
Reviews stated that migration distances vary from <1 mile up to nearly 100 miles (2-160 km) [135,215]. Migrations may take from 4 to 7 weeks [7]. In the Green River Basin, mule deer took 9 to 13 weeks to complete their migration in spring and fall, spending 4 to 5 months each year on midelevation transitional ranges [285].
Fawn mortality is generally higher than that of adults. It is often highest at or immediately following parturition [58,77,77,163,215,283]. According to a review, 25% to 30% of fawns are commonly dead by fall, 50% more by early winter, and up to 75% or more by spring [215]. For example, in the declining North Kings River mule deer herd in oak-grassland and chaparral habitats in California, about 50% to 70% of fawns died within the first month of life, whereas winter fawn losses were "minor". The largest cause of mortality was coyote predation. The authors hypothesized that low fawn recruitment and population decline were due to reduction in the occurrence of fire and other disturbances on summer and transitional ranges that led to a decline in nutritional quality of mule deer forage during the last trimester of pregnancy and the lactation period [283].
Fawn survival may be related to fawn weight and gender, litter size, and other factors. In Colorado, Idaho, and Montana, where the average annual mortality of mule deer fawns was 66%, the heaviest fawns at the start of winter had the highest overwinter survival (P<0.001). Predation and malnutrition accounted for most deaths [338]. On 3 winter ranges in southwestern Idaho, the probability of winter fawn mortality increased with lower body mass (P=0.007) and being male (P=0.018) [31]. In the scablands of eastern Washington, twin fawns had a risk of dying 2.6 times higher than that of single fawns [163]. In north-central New Mexico, mule deer fawn survival was related to birth mass, birth date, litter size, maternal body fat, and total and seasonal precipitation (P<0.009 for all variables). The authors concluded that fawn survival was driven in part by an interaction between total and seasonal precipitation and effects of these factors on plant production, with consequential effects on female nutrition, and ultimately, fawn birth attributes [198].
Weather may affect fawn survival. In several plant communities in central Oregon, mule deer fawn survival during winter was closely related to temperature, wind, and snow cover and depth. Fawn survival decreased as the combination of these factors increased in severity (Leckenby and Adams 1986 cited in [106]). In Prairie County, Montana, there were significant relationships between the total amount of precipitation occurring from July to April prior to fawning and percent of fawns in the population the following winter (r=0.75, P=0.01) and spring (r=0.76, P=0.01). Average or greater precipitation during summer apparently resulted in relatively good summer forage conditions during those years, leading fawns to have ample fat reserves that could carry them through even severe winters. On the other hand, fawn winter mortality rates were relatively high in years with extreme drought and resultant poor summer forage, even when winters following the drought were mild [365].
Several other studies reported relationships between precipitation or forage production on summer ranges and herd productivity or population fluctuations (e.g., [129,164,250,303]).
Cover is important in fawning areas. Cattle (Bos primigenius) grazing may result in a loss of hiding cover for fawns, possibly increasing fawn predation mortality [197]. See Livestock grazing for more information.
Malnutrition is often the leading cause of mule deer deaths. On Utah juniper-big sagebrush rangelands in southeastern Utah, winter mule deer mortality varied inversely with the amount of available browse [277]. Prolonged, continuous snow cover may result in substantial mortality due to malnutrition and starvation [78,88]. For example, in Oregon, a mule deer die-off occurred after snow covered the Cedar Creek Enclosure for >50 consecutive days; the die-off occurred about 23 years after the last wildfire [148].
In the prairies of the northern Great Plains, annual variations in amount and timing of precipitation influence vegetation production and thus mule deer mortality due to malnutrition [365]. In Prairie County, Montana, overwinter fawn mortality rates of mule deer were positively correlated with winter severity during 4 of 12 years (r=0.94, P=0.03). During the other years, fawn survival rates appeared to be mainly influenced by drought and poor forage the prior summer. Total amount of precipitation occurring in the area from July to April prior to fawning and percent of fawns in the population in spring were positively correlated (r=0.76, P=0.01) during the 12 years [365].
In the arid Southwest, precipitation may indirectly affect mule deer mortality through its effects on plant productivity [217,219,347]. A study in the Sonoran Desert of California found positive correlations between rainfall and the proportion of mule deer in good physical condition (r=0.60, P=0.064) and fair physical condition (r=0.70, P=0.017), whereas the proportion of mule deer in poor physical condition was negatively correlated with rainfall (r= -0.72, P=0.20) [218]. In the Trans-Pecos region of Texas, abundance of adult mule deer (R=0.645, P≤0.001) and fawn production (R=0.553, P≤0.003) were correlated to the Palmer Hydrologic Drought Index, indicating that the mule deer population was negatively affected by drought [352]. At Three Bar Wildlife Area in Arizona, survival of mule deer fawns in a given year varied with total rainfall during the previous winter. This relationship appeared to result mainly from the influence of precipitation on production of winter-growing forbs: Variation in forb production accounted for about 75% of the total variation in fawn survival during the 8-year study [303]. A severe, year-long drought in desert grassland of southeastern Arizona caused an apparent decline in local mule deer and white-tailed deer populations [11].
Black-tailed deer may have high mortality during hot, dry summers in California chaparral [14,90,323,324]. In this habitat, there is typically an abrupt decline in forage quality in June and July as vegetation desiccates. During dry summers, black-tailed deer mortality may be high if forage desiccates early, while fawns are nursing. In addition, years of low acorn production may lead to high mortality in August and September. Conversely, because the acorn drop is coincident with the breeding period, years of good acorn production may improve breeding conditions of bucks and does, resulting in increased fawn production [14,323].
Disturbance can produce habitat for mule deer by favoring forage growth and by creating ecotones between areas of dense cover and more open feeding areas. Conversely, loss of cover over large areas can be detrimental to mule deer [135,215]. Several researchers suggested that resource managers may need to consider proximity of food, cover, and water before implementing actions that may impact mule deer habitats [153,215,216].
Prescribed fire: For information on the use of prescribed fire in mule habitats, see Fire Management Considerations.
Logging: With the exception of black-tailed deer in British Columbia and southeastern Alaska, which are dependent upon canopy cover in mature forests [134,135,215,325,350], mule deer generally benefit from early successional vegetation that establishes after logging and other disturbances [135,215]. Logging may benefit mule deer because early-seral habitats often contain a greater variety, quantity, and quality of mule deer forage than mature forests (e.g., [39,72,98,137,269,290,304,310,325,349]). However, forage quantity and quality may not increase immediately in logged areas and may last only 20 to 30 years (e.g., [134,147,215,269,310,350]). In addition, cover may be reduced [310]. A review stated that clearcutting of old-growth forests in southeastern Alaska has 4 potential effects may decrease the carrying capacity of habitat for black-tailed deer: 1) sun-grown plants in open clearcuts may have lower digestible protein concentrations than shade-grown plants in forests; 2) large amounts of logging slash may increase energy costs of locomotion for black-tailed deer and reduce the area of usable habitat; 3) snow may accumulate and persist more in open clearcuts than in forests; and 4) understory production may be reduced to extremely low levels when the conifer canopy closes; this may occur at about 20 to 30 years after logging and persist for 100 years or more [134].
In general, the duration of logging benefits to mule deer varies with forest type, soils, climate, and other factors. Use of prescribed fire, herbicides, soil scarification, planting of seeds and seedlings, and other site preparation may shorten or lengthen the time a logged site is used by mule deer [134,147,215]. For example, burning young clearcuts in southeastern Alaska may benefit black-tailed deer by reducing shrub and conifer biomass and increasing the diversity of herbaceous forage plants, thus potentially delaying conifer canopy closure [134]. In addition, succession following clearcutting may be affected by heavy mule deer browsing. For more information, see Mule deer foraging effects. Mule deer use of logged areas is modified by opening size, logging slash, predisturbance movement patterns, and weather, particularly snow depth. Reviews of logging effects on mule deer are available: [72,134,351].
Opening size: The size and distribution of clearcuts in space and time are important to mule deer; this is likely also true of burned sites. In the boreal forest zone in western Alberta, the size and dispersion of 2- to 9-year-old clearcut blocks and type of treatment best explained mule deer and white-tailed deer use of clearcuts (R²=0.21, P<0.01). Deer showed a strong preference for clearcut blocks that were <40 acres (16 ha); because they preferred areas within clearcuts that were <330 feet (100 m) from cover, they favored configurations that provided a high degree of edge per unit area. They also preferred clearcuts that were scarified or scarified and burned under prescription compared with untreated clearcuts. The authors suggested that such treatments may have led to greater abundance of preferred herbaceous species and reduced logging slash, which benefited deer. Clearcut blocks in clumped patterns appeared unfavorable [334]. Some authors suggested that many small, scattered, irregularly shaped clearcuts may be preferable to fewer, large, block-shaped clearcuts because multiple small treatments would contact the home ranges of more mule deer [135,334]. A review stated that deer used natural and created openings in ponderosa pine forests similarly, particularly when thinned stands occurred nearby, but in dense stands, deer likely benefited from small openings [72]. For more information, see Edge habitat. See the review by Wallmo and Schoen [351] for management recommendations regarding sizes of clearcut openings in various regions.
Logging slash: Depending upon its density, logging slash may be a benefit or a detriment to mule deer. Reviews stated that abundant logging slash generally impedes mule deer movements and may act as a barrier to mule deer use of clearcut openings and selectively logged areas [63,135,351]. Conversely, some logging slash can provide cover for mule deer [351]. In quaking aspen stands on the Apache and Coconino National Forests, Arizona, deer use was lower in thinned quaking aspen stands without slash removal despite greater density of perennial grasses, forbs, and quaking aspen sprouts in these stands compared to unthinned stands. Apparently, the amount of woody debris in thinned stands prohibited deer use [268]. In southeastern Alaska, dense logging debris apparently impeded black-tailed deer use of 1- to 2-year-old clearcuts in western hemlock-Sitka spruce forest. Dense logging debris continued to impede black-tailed deer until 15 to 20 years after logging [350]. Mule deer pellet group counts in clearcut strips in subalpine lodgepole pine and spruce-fir forest in Colorado were less than those on adjacent uncut sites during the first year after logging, possibly due to the deep tangle of residual slash and disturbance of logging operations. However, 10 years after strip clearcutting, pellet group counts were 2 times higher on clearcut strips than on adjacent uncut strips [344]. In a selectively cut ponderosa pine forest in Arizona, deer pellet groups were more numerous where slash was undisturbed after logging. Slash abundance was 1.7 times greater where slash was undisturbed than where it was piled and burned, but forbs were more abundant where slash was piled and burned, which should have attracted deer. The author suggested that deer may have preferred the site where slash was undisturbed because the slash provided protective cover [266]. In north-central Arizona, mule deer use was higher on clearcuts where the slash had been piled and burned than on clearcuts where slash had been piled but not burned [189]. In Arizona, Neff (1980 cited in [72]) found that deer showed no preference for either the presence or absence of slash in small (1-10 acres (0.4-4.0 ha)) openings in ponderosa pine stands.
A review recommended prescribed fire in southeastern Alaskan forests after clearcutting to reduce logging slash, reduce shrub biomass, favor herbs, reduce conifer regeneration, and prolong the useful life of clearcuts for mule deer, at least in snow-free seasons or areas [135]. In juniper woodlands in Texas, Bryant [57] suggested that 10% to 15% of cleared areas should contain slash to provide cover for mule deer. Black-tailed deer may also benefit from reduced logging slash that potentially impedes their movements [134]. For more information, see Indirect Fire Effects.
Logging and weather interactions: Mule deer may not use clearcuts because of deep snow compared to forests [357]. In the interior western redcedar-western hemlock subzone near Horsefly, British Columbia, mule deer tracks during a year of low snowfall were half as abundant in clearcuts as in uncut forest. Snow was 17 inches (44 cm) deep in openings and just 10 inches (26 cm) deep in forests, suggesting that deep snow in clearcuts may have reduced forage access and thus use of clearcuts [357]. For more information, see Foraging sites.
Other treatments:
Sagebrush and pinyon-juniper: Removal of shrubs and trees in sagebrush and pinyon-juniper ecosystems is a common management practice on mule deer rangelands. In sagebrush and pinyon-juniper ecosystems, large areas have been treated mechanically, with prescribed fire, or with herbicides to try to convert them to grass-shrub or grass types [66,347]. Such treatments in sagebrush communities may reduce important winter forage for mule deer [91]. Partial or complete removal of trees in pinyon-juniper communities may result in substantial increases in production of grasses, forbs, and shrubs, which could potentially increase mule deer carrying capacity [66], but according to a review, this practice produces mixed results. Some studies showed increased mule deer use of treated pinyon-juniper sites, typically due to greater amounts of forage and browse on treated areas, while other studies did not show increased mule deer of treated areas, typically because of reduced cover in these areas [2]. On the Zuni Indian Reservation in western New Mexico and eastern Arizona, mule deer pellet groups increased with the number of pinyon and juniper trees removed (R²=0.95, P=0.03) [2]. A similar increase did not occur on 2- to 24-year-old pinyon-juniper rangelands in Utah that were chained and seeded with grasses, forbs, and shrubs. The authors suggested that treated sites were used despite decreased cover because of increased forage [299]. At Fort Bayard, New Mexico, mule deer abundance in Colorado pinyon (Pinus edulis)-juniper habitat was higher before than after tree removal. Mule deer forage increased after tree removal, but the authors concluded that the absence of cover reduced mule deer use [294]. In a review, Phillips [256] stated that chained pinyon-juniper stands did not benefit mule deer and other wild ungulates until trees and shrubs established [299]. Other researchers reported that treatment of pinyon-juniper rangelands did not affect mule deer habitat use. Mechanical and herbicide treatments on 5,200 acres (2,100 ha) of pinyon-juniper rangeland in Arizona resulted in no differences in mule deer use of the area (Neff 1980 cited in [290]).
Mule deer use pinyon-juniper woodlands in all stages of succession [2]. How long each stage is utilized depends in part on site, composition of the understory prior to disturbance, the type of disturbance, weather conditions, postdisturbance treatments such as seeding, and livestock grazing [290]. In general, the usefulness of pinyon-juniper habitats to mule deer declines as the understory and midstory decline [295]. Based upon studies in west-central Utah, posttreatment production of forbs and grasses generally diminishes to pretreatment levels in <20 years; shrubs increase up to 40 years after treatment; and at 40 years, juniper and pinyon again dominate the site [21]. In Nevada, annual and perennial forbs dominated for 1 to 2 years after canopy removal; perennial grasses dominated in the 2nd year and reached maximum abundance in the 4th year. Shrubs reached a peak after grasses, between the 1st and 3rd posttreatment years, and trees regained dominance in <15 years [329].
According to a review, published literature is "nearly unanimous" in recommendations for pinyon-juniper management for mule deer and other wild ungulates: 1) keep openings small and close to escape cover, usually 0.1 to 0.2 mile (0.16-0.32 km) maximum; 2) locate projects near areas of historical big game usage; and 3) leave browse plants untreated or reestablish following treatments [2]. These authors provide management recommendations for mule deer and other ungulates in pinyon-juniper communities: [57,290,295]. See Great Basin woodlands for information about fire effects on mule deer in pinyon-juniper communities. For a review of the effects of management practices in sagebrush steppe on mule deer—including topics not discussed here such as management of sagebrush with herbicides, fertilizing sagebrush habitats, and reseeding after sagebrush reduction—see Carpenter and Wallmo [66]. See Great Basin shrublands for information about fire effects on mule deer in sagebrush communities.
Gambel oak: Gambel oak is an important mule deer forage species; both its mast and browse are used extensively. It may form almost pure stands in some areas. Because of its growth habit, however, it often forms impenetrable thickets that are too tall or inaccessible for mule deer [66,186]. Methods used to treat Gambel oak communities include prescribed fire, logging, and herbicides. Clearcutting patches in Gambel oak habitat may produce abundant browse because of Gambel oak's sprouting ability, but this would temporarily reduce acorns. Selective cutting, in which the best acorn-producing trees are left, was recommended by Severson and Medina [290] to ensure both browse and acorn production at a single location. A review stated that treating Gambel oak stands with prescribed fire or mechanical methods may increase mule deer use of treated stands up to 4 times but that use declines as time since treatment increases and stands become dense and inaccessible [186]. In Colorado, dense Gambel oak stands were sprayed with herbicides. Two years after spraying, grasses increased 44% compared with pretreatment levels, while shrubs decreased 29% and forbs decreased 15%. Five years after spraying, grasses were 17% below pretreatment levels and shrubs were 7% below pretreatment levels. Consequently, herbicide treatment was considered beneficial to mule deer for only a brief time, and frequent retreatment was considered necessary to maintain high-quality habitat for mule deer [180]. See Southwest shrublands for information about fire effects on mule deer in Gambel oak communities.
Livestock grazing: Influences of livestock grazing on mule deer can be detrimental, neutral, or beneficial [67,153,216,290]. Grazing, as well as the physical presence of cattle, domestic sheep (Ovis aries), and other livestock can have negative impacts on mule deer not only by reducing forage and changing ratios of live to dead plant material [8,153,290,361,362], but by causing changes in movements and behavior and altering activity budgets [67,67,169,216,263,290]. However, some researchers reported few or no effects of livestock grazing on mule deer in areas where livestock grazing intensity was low or moderate [18,67,153,290]. Livestock grazing in many perennial grasslands historically increased shrub and annual grass-forb types, potentially benefiting mule deer (see Threats) [153,216,290]. In other areas, heavy livestock grazing reduced shrubs and herbs important as mule deer forage [216]. Reviews noted that livestock grazing on the northern Great Plains has extensively reduced or eliminated hardwood tree and shrub cover along drainageways, which has limited the occurrence of mule deer in some areas [287,290]. In the Sierra Nevada, cattle grazing during peak fawning season reduced hiding cover for fawns in both quaking aspen and meadow-riparian habitats compared with areas without cattle grazing [169,197]. Reviews stated that livestock management practices and factors that may affect mule deer include weather, topography, water availability, rangeland type, grazing intensity, animal distribution, livestock species, grazing system, and timing and duration of grazing. Timing of livestock grazing was considered particularly important to mule deer, which are particularly susceptible to adverse effects during fawning [17,67,153,169,215,216,290,290].
Water management: On some arid ranges of the Southwest, water development and better distribution of water sources for livestock can benefit mule deer by permitting year-round or seasonal use of rangelands from which they may have been excluded by a lack of free water (see Water) [153,215,216,280]. Mule deer numbers increased during the 5 years following development of permanent water sources on areas of Fort Stanton, New Mexico, that had little or no free water previously. In one area, use increased from <1.6 to >13 mule deer/mile² in 5 years. In another area, use increased from 14.2 to 19.2 mule deer/mile² in 1 year, dropped to 9.4 mule deer/mile² in the 4th year when water sources deteriorated, and increased to 22.1 mule deer/mile² in the 5th year when water was again available [367]. However, water developments may concentrate livestock and deer, leading to degradation of some habitats [153,215,216]. Furthermore, redistribution of livestock through water development may increase overlap of mule deer and livestock on areas previously occupied by mule deer but not livestock [153,216]. For more information, see the review by Mackie and others [215].
Mule populations were reduced substantially following European settlement. By 1900 populations had declined due to overgrazing by livestock, overharvesting, drought, agriculture, and land development [30,287,340]. From the 1910s to the 1950s, however, widespread logging, fire, and predator reduction programs benefited mule deer, and populations in some areas were higher than they had been historically [340]. Encroachment of woody plants onto some areas of the Southwest and Great Basin that were formerly dominated by grasses due to livestock grazing, alterations of fire patterns and fire exclusion, and possibly climatic shifts, have increased winter cover and forage for mule deer [17,127,216,290,340]. Increases of woody plants were followed by dramatic increases in mule deer populations [17,340]. However, such changes also resulted in reduced summer rangelands, and intensive cultivation and development further reduced habitats for mule deer [340]. By the 1940s and 1950s, many mule deer populations exceeded carrying capacity [330,340]. In the mid-1960s and early 1970s, mule deer populations declined sharply over much of the United States [201,340]. The decline was attributed to habitat losses from urbanization and other land development, fire exclusion, vegetation succession, conversion of shrublands to grasslands, and deterioration of winter ranges due to excessive use by large ungulate populations [153,201,340]. In some areas, repeated wildfires have resulted in the conversion of native shrub-grass habitats to environments dominated by nonnative invasive plants such as cheatgrass (Bromus tectorum). These changes have had substantial negative impacts on mule deer [330]. Beginning in the late 1970s and early 1980s, mule deer numbers continued to decline in some areas but remained stable or increased in others [340].
Threats to mule deer populations include overharvesting, increased human disturbance, and nonnative invasive plants:
Human disturbance: Mule deer may habituate to human presence and become nuisances in some areas. However, human development generally reduces mule deer use of developed areas [215,347].
Nonnative invasive plants: Spread of nonnative invasive grasses and forbs may harm or benefit mule deer. Some researchers found that mule deer commonly consume nonnative invasive plants, including spotted knapweed (Centaurea maculosa) (e.g., [50,105,190,206,244,270,278,368]). Along the Selway River in Idaho, mule deer ate spotted knapweed seedheads, particularly when snow was on the ground, because the seedheads were easily obtainable above the snow. In fact, they were one of the few herbaceous plants readily available to mule deer in open areas when snow was >12 inches (30 cm) deep. Mule deer also ate large amounts of spotted knapweed rosettes, particularly in spring after snowmelt [368]. Mule deer consumed green shoots of cheatgrass and rosettes of tumble mustard (Sisymbrium altissimum) in late winter and spring after several wild and prescribed fires in Lava Beds National Monument, California [261].
Some sources suggested that the carrying capacity of rangeland for mule deer and livestock may be reduced by nonnative invasive plants that displace more palatable native grasses and forbs (e.g., [46,105,206,271]). In the Bitterroot Valley, Montana, mule deer rarely used spotted knapweed-dominated open areas [360]. However, along the Selway River in Idaho, where densities ranged from 0.03 to 0.17 mule deer/ha during winter, spotted knapweed infestations of xeric south and west-facing slopes on year-round rangeland did not appear to affect mule deer carrying capacity in winter when compared with Saskatoon serviceberry/bunchgrass-sedge shrubfields [368]. Wright and Kelsey [368] attributed differences between the studies to lower mule deer densities, greater availability of agricultural lands, and less snow cover in the Bitterroot Valley study.
Mule deer may contribute to the spread of nonnative invasive plants by ingesting, transporting, and disseminating viable seeds of nonnative invasive plants in their feces [25,89,139,240,244,342,343]. In maritime chamise-La Purissima manzanita (Arctostaphylos purissima) chaparral habitats in Santa Barbara County, California, mule deer dispersed seeds of hottentot fig (Carpobrotus edulis), a nonnative invasive plant, into recently burned areas [89].
The spread of cheatgrass has important indirect effects on mule deer and other wildlife by increasing fuel loads and fire frequency, which may alter the structure and composition of native plant communities. Because sagebrush communities provide important winter rangelands for mule deer and sagebrush is easily killed by fire, cheatgrass invasion may be particularly detrimental to mule deer in sagebrush habitats [270,371]. Buildup of medusahead (Taeniatherum caput-medusae) litter may also lead to increased fuel loads and more frequent fires in low sagebrush (Artemisia arbuscula) and other sagebrush communities [271]. For more information, see FEIS reviews of cheatgrass, medusahead, and other species of interest.
Mule deer require cover for security, thermal protection, and snow interception [34,58,102,119,215,347]. Cover influences the energetic costs of maintaining body temperature; the abundance of forage; security from predators and humans; and the costs of movement through snow [58]. According to a review, concealment cover is provided by vegetation within 7 feet (2 m) of the ground. Olson [241] described patches of concealment cover as "any vegetation capable of hiding 90% of a mule deer from human view at a distance ≤200 feet (60 m)". Conifers and other evergreen plants provide some of the best cover for mule deer in winter [241,287]. Topographic features such as boulders, river breaks, irregular topography, and ledges also provide concealment cover for mule deer [58,253]. Thermal cover is provided by vegetation and topography that ameliorate temperature and wind. During high wind, mule deer seek pockets of calm air below the crests of hills and in dense forests [102,119]. The importance of thermal cover varies with season, weather, and the age, size, and nutritional condition of the animal [58]. Based upon a simulation model using data from 14 years in shrubsteppe and shrub-woodland winter ranges in Colorado, mule deer doe and fawn thermal cover requirements under severe winter weather conditions were inversely correlated with the physical condition of the animal. However, under less than severe weather conditions, enhancing thermal cover on shrubsteppe and shrub-woodland winter ranges appeared unlikely to improve mule deer condition, although loss of cover could markedly alter patterns of mortality [150].
Snow interception cover is provided by forest and shrub canopy cover and topography. According to Bunnell [58], the degree of canopy closure has the most influence on the proportion of snowfall that will be intercepted by a forest >30 feet (10 m) tall. Forest stands with high canopy closure have shallower snow beneath, which reduces the cost of movement and increases forage availability [58]. In coastal British Columbia, mean snow depths across 4 mountain hemlock stands of different ages decreased linearly with mean canopy closure (r²=0.87, P<0.05). The stands included a 200-year-old forest with 60% canopy closure, an 80-year-old stand with 90% canopy closure, a 20-year-old stand with 36% canopy closure, and a recently clearcut stand with 0% canopy closure. The 2 oldest stands had less snow and a harder crust than the recent clearcut. Thus, mean black-tailed deer sinking depths were lower in those stands [60]. Clearcuts in western redcedar-western hemlock-western white pine forests in northern Idaho accumulated more snow than mature forest. However, spring snow melted faster from clearcuts than mature forest. Clearcuts on south-facing slopes had exposed forage soonest in spring [138]. A sagebrush canopy intercepts snow in addition to providing frequently melted-out areas around large plants, allowing for access to forage. Near Dubois, Wyoming, big sagebrush stands with about 50% cover accumulated more snow than open grasslands. In spring, snowmelt began earlier and proceeded at greater rates in and adjacent to big sagebrush crowns [158]. Because the amount and duration of snow varies within and among years, the value of canopy cover to mule deer also varies [253].
Mule deer are attracted to canopy openings by abundant forage but may make little use of the centers of large openings because of distance from cover [351]. See Edge habitat for more information.
Mule deer forage-site selection is based in part on forage quantity and nutritional quality, which are influenced by plant species composition, plant phenology and related changes in nutrition, site characteristics (soil, shade, and topography), successional stage, grazing and browsing pressure (see Livestock grazing), and weather. Mule deer forage-site selection is also affected by predation risk and proximity of foraging sites to drinking water and habitats providing cover.
Weather affects mule deer forage availability and thus foraging-site selection throughout the year. For example, black-tailed deer along the northern Pacific Coast in British Columbia and southeastern Alaska largely depend on canopy cover in mature forests [135,347,350]. A review of black-tailed deer habitats in southeastern Alaska, where black-tailed deer used forests year-round, stated that the 2 most important features of forest vegetation for black-tailed deer are a productive understory of high-quality forage and an overstory that intercepts and/or redistributes enough snow that understory forage remains available throughout the winter [135]. Patterns of forest use by black-tailed deer in southeastern Alaska shift through the winter and spring with changes in snow conditions and plant phenology [135]. In high snow areas in British Columbia and Alaska, "critical" winter rangelands include areas at low elevations; areas with southern aspects on moderate to steep (40%-100%) slopes; forests with multiple canopy layers; small, interspersed openings; and dense forest patches with well-developed crowns that intercept snow [58]. Black-tailed deer in areas of deep snow along the northern Pacific Coast largely depend upon old-growth western hemlock-Sitka spruce stands of moderate to high volume (≥20,000 board feet/acre) with an understory of huckleberry, bunchberry, and strawberryleaf raspberry (Rubus pedatus) due to the high degree of snow interception by the canopy and high-quality forage in the understory. In areas of low snow, however, forest stands with more open canopies and lower densities may be relatively more important [135]. In spring, the most important habitats for black-tailed deer are those with early snowmelt because these areas provide abundant, early succulent vegetation. Open areas such as clearcuts, rocky outcrops, and open forests have rapid snowmelt and early initiation of spring growth [58]. Wet sites, particularly those with patches of skunk cabbage (Lysichiton americanus), also provide abundant early new growth and are important spring foraging habitats [135].
Mature chaparral stands provide essential cover and forage for mule deer during parts of the year [345]. Mule deer summer foraging sites in California chaparral include riparian areas, seeps, springs, streams, and ponds. In fall, foraging sites include stream bottoms, ridge tops, and northern slopes. In winter, mule deer forage on south slopes and sheltered ridges [14]. A review stated that mule deer carrying capacities in chaparral are largely determined by weather and its effects on forage quality and quantity. "Good deer years" have weather conditions that promote herbaceous forage production and acorn production and/or extend forage succulence through the summer and fall [14]. Diversity of habitats is important to mule deer in California chaparral. Biswell [39] stated water availability, combined with chamise chaparral on south-facing slopes and mixed chaparral on north-facing slopes and in drainages, favored black-tailed deer in Lake County, California, because the combination provided diverse browse. In California oak woodlands, black-tailed deer use all successional stages, but Anderson and Pasquinelli [10] considered oak woodlands with abundant seedlings and saplings most important.
A review stated antipredator strategies of mule deer include early detection and outmaneuvering of predators; avoidance of areas frequented by predators; formation of groups with other mule deer; and restriction of movements to areas close to cover or escape terrain (e.g., steep slopes, riverbanks, and areas with low obstacles such as deadfall). Mule deer may also defend themselves against predators such as bobcats and coyotes [119,121]. Geist [121] suggested that the mule deer's antipredator strategies in part determine the species' preference for areas with broken terrain and steep slopes with obstacles.
Presence of predators may alter mule deer habitat use, movements, diet, and behavior (e.g., [4,239,258]). At the Three Bar Wildlife Area, summering female mule deer in enclosures with coyotes used areas with denser vegetation than females in enclosures without coyotes. Four habitats occurred in the enclosures: burned and unburned interior Arizona chaparral and burned and unburned Sonoran desertscrub. Burns resulted from a severe spring wildfire 4 years earlier. Vegetation was denser in burned and unburned chaparral than in desertscrub. Thus, mule deer may have selected chaparral for its escape and hiding cover from coyotes. However, they may have selected it for its greater thermal and security cover or for its greater forb biomass [239]. In the eastern Sierra Nevada, where mountain lions accounted for 68% of predator-caused mortality, male and female mule deer selected locations at higher elevations with more antelope bitterbrush, an important winter forage species, than random locations. Mountain lion kill sites were in relatively more open locations than locations in which mule deer foraged (P<0.05 for all variables). Therefore, mule deer did not appear to be confronted with a trade-off between predation risk and forage abundance when selecting habitat [258].
Predation risk from human hunting may also alter mule deer habitat use, movements, diet, and behavior [78]. For example, near Fort Collins, Colorado, hunting resulted in mule deer moving to areas with dense cover within their home ranges [182].
Gestation ranges from 183 to 218 days [7,58,215]. In the northern part of the mule deer's distribution, parturition occurs primarily from late May to mid-July [215]. In the southern part, parturition occurs primarily in July and August [215,290]. Extreme birth dates occur as early as mid-May and as late as early October [7]. Like the rut, fawning periods tend to occur within a short period. In Utah (Robinette and others 1977 cited in [5]) and Colorado (Anderson and Medina 1967 cited in [5]), for example, about 85% of fawns were born within a 32-day period. However, a long fawning period may be more typical in arid areas of the Southwest where rainy seasons are unpredictable. In Arizona, fawning coincided with the summer monsoon season and in one study ranged from 5 August to 5 October [115].
Growth: As parturition approaches, pregnant females move to fawning areas. Does with fawns may remain in these areas until late summer or fall [215]. Fawns weigh from 4.5 to 11 pounds (2.0-5.0 kg) [5,7,215]. Singletons weigh more than fawns from litters with ≥2 fawns [5]. Males and females tend to weigh the same at birth [5], although among sets of twins of opposite sex, males may be heavier [7]. A fawn's birth weight may affect its survival. A review stated that the effect of a maternal doe's physical condition on fawn birth weights is unclear. However, females in good physical condition the year before parturition may have a shorter gestation and/or give birth earlier than females in poor physical condition [215].
Newborn fawns hide and may be separated from their mothers for long periods. The hiding period lasts for 6 to 8 weeks [119]. Fawns begin to consume green vegetation at 2 weeks old and are weaned in fall, when their mothers breed again [7,14,102,215]. Young remain with their mothers until the following spring, when their mothers drive them away before giving birth [215]. For more information, see Social behavior.
Fawns grow rapidly, with males tending to be heavier than females. Yearling females weigh about 10% less than yearling males on average [215]. Anderson and others [6] concluded that male mule deer on the Roosevelt National Forest continued to gain weight throughout their lives, whereas females reached their maximum body weight at about 8 years old. In contrast, Mackie (1964 cited in [215]) concluded that males in Montana gained weight until at least 7.5 years old, whereas female weight changed little after 2.5 years old.
Most mule deer attain sexual maturity and can breed as yearlings [215]. However, yearling males are frequently prevented from mating by older males [215]. Fawns may become pregnant, but this is rare in the wild [215,322]. The age at first parturition is influenced by nutritional condition. In severely malnourished populations, the age at first parturition may be ≥3.5 years old [215].
Pregnancy and twinning rates: Adult mule deer commonly produce twins, whereas yearlings usually produce singletons; triplets and quadruplets are rare [5,77,215]. Pregnancy rates are influenced by local environmental conditions and nutritional status of does. In the Missouri River Breaks region of Montana, mule deer produced an average of 44% singletons, 55% twins, and 1% triplets during 12 years. During times with the poorest range conditions, mule deer produced 75% singletons, 25% twins, and no triplets. During times with the best conditions, they produced 27% singletons, 70% twins, and 3% triplets [129]. According to a review, triplets are born mainly to >4-year-olds [5].
Pregnancy rates range from 70% to >90% among adult (≥1.5-year-old) females. Yearlings typically have lower pregnancy rates than adults [215]. At Hopland Research and Extension Center, California, wild black-tailed deer fawns bred when the population was experimentally reduced from 25 to 10 black-tailed deer/km². The author suggested that the reduced population density resulted in rapid body growth and early maturation [227]. In the Missouri River Breaks region, 4-, 5-, and 6-year-old mule deer females were the most productive segment of the population. Reproduction declined sharply at 7 years old. Females ≥8 years old had reproductive levels similar to that of 3-year-olds, which were 12% to 21% lower than levels of 4-, 5-, and 6-year olds [130]. Reproduction in males may decline at 7 years old [5].
The number of fawns per doe varies with range quality [293]. The number of mule deer fawns per doe on "poor" Utah juniper/big sagebrush rangeland in south-central Utah was 64% of that on "good" Utah juniper/big sagebrush rangeland in southern Idaho [164]. In California chaparral, the black-tailed deer fawn:doe ratio was <85:100 in dense, tall chaparral. It was 147:100 in areas where repeated prescribed fires and seeding of grasses and legumes resulted in a mosaic of grasslands with scattered areas of dense chaparral, and it was 116:100 in an area burned in a summer wildfire 2 years previously that had large areas of small shrubs and very little herbaceous cover [320,322]. Taber [323] suggested that a diet low in protein and phosphorus resulted in low ovulation and reproductive rates of black-tailed deer living in the dense chaparral.
In the arid Southwest, the abundance of forage is frequently controlled by seasonal rainfall, and Hungerford [156] suggested that precipitation during the summer may be the most important factor regulating mule deer fawn production and survival. On the Kaibab Plateau in Arizona, a dry summer resulted in the lowest fawn production on record (28 fawns:100 does) [319]. In another study, fawn production on the Kaibab National Forest, north-central Arizona, averaged 90% 5 years after about 10% of the mule deer summer rangeland was treated. Slash piles were burned and grasses, legumes, and shrubs were seeded in forested areas. Some forested areas were also logged. Meadows, which were historically overgrazed, were disked and seeded. Fawn production during the 5 years prior to treatments averaged 66%. The author suggested the increased fawn production was a result of increased forage productivity due to the treatments [156].
The scientific name of mule deer is Odocoileus hemionus (Rafinesque) (Cervidae) [363]. Mule deer and black-tailed deer comprise 2 groups. The mule deer group has as many as 7 subspecies, and the black-tailed deer group has 2 subspecies [215]:
Mule deer group:
Odocoileus hemionus subsp. hemionus (Rafinesque), Rocky mountain mule deer [121,215,363]
Odocoileus hemionus subsp. californicus (Caton), California mule deer [121,215,363]
Odocoileus hemionus subsp. cerrosensis Merriam, Cedros Island mule deer [363]
Odocoileus hemionus subsp. eremicus (Mearns), desert mule deer [215]
Odocoileus hemionus subsp. fuliginatus (Cowan), southern mule deer [121,215,363]
Odocoileus hemionus subsp. peninsulae (Lydekker), peninsula mule deer [121,215]
Odocoileus hemionus subsp. sheldoni Goldman, Tiburon Island mule deer [121,363]
The taxonomic status of the Cedros Island [215,347] and Tiburon Island [121,215,347] mule deer is in doubt, and the Inyo mule deer (O. hemionus subsp. inyoensis (Cowan)) [121,363] is generally no longer recognized as a distinct subspecies [215,347].
Black-tailed deer group:
Odocoileus hemionus subsp. columbianus (Richardson), Columbian black-tailed deer [121,215,363]
Odocoileus hemionus subsp. sitkensis Merriam, Sitka black-tailed deer [121,215,363]
Subspecies are distinguished by body size, pelage color, skull form and dentition, size and shape of antlers, behavior, and geographical distribution [5,119,121,215,347]. However, the distinction of North American subspecies has been brought into question by genetic analyses. Cronin and others [84] found variation in mitochondrial DNA between mule deer and black-tailed deer groups but not between Columbian black-tailed deer and Sitka black-tailed deer. Translocations have led to intermixing of subspecies in some areas [347], and subspecies may interbreed where they coexist [83,84]. See Geist [121] for more information about subspecies distinctions.
Mule deer and white-tailed deer (O. virginianus) may hybridize where their ranges overlap [83,85,155,316], although hybrids are rare in the wild [121]. The survival of hybrids in captivity [7] and in the wild [121] is poor. For more information about mule deer and white-tailed deer hybridization, see Geist [121].
This review synthesizes information about mule deer and black-tailed deer at the group level, when possible. Collectively, they are referred to as mule deer throughout this review. In some publications the term "deer" was used to describe mule deer and white-tailed deer in combination. In those cases, this review does the same.
Ar c'harv mul (Odocoileus hemionus) a zo ur c'harv hag a vev e kornaoueg Norzhamerika.
a vo kavet e Wikimedia Commons.
Ar c'harv mul (Odocoileus hemionus) a zo ur c'harv hag a vev e kornaoueg Norzhamerika.
El cérvol mul (Odocoileus hemionus) és una espècie de cérvol. Deu el seu nom a les seves grans orelles, que s'assemblen a les d'un mul. És l'espècie més gran del gènere Odocoileus i el seu hàbitat és a la part occidental de Nord-amèrica.
El cérvol mul (Odocoileus hemionus) és una espècie de cérvol. Deu el seu nom a les seves grans orelles, que s'assemblen a les d'un mul. És l'espècie més gran del gènere Odocoileus i el seu hàbitat és a la part occidental de Nord-amèrica.
Jelenec ušatý (Odocoileus hemionus) je přežvýkavec z čeledě jelenovitých (Cervidae), který žije na západě Severní Ameriky. Jedná se o největšího zástupce z jelenců. Jelenec ušatý má typické, mule podobné, velké uši, od kterých je odvozen jeho druhový název.
Je dlouhý 1-1,9 m, váží 50-215 kg a ocas má 10-25 cm dlouhý. V zimě má šedohnědě, v létě zas červenohnědě zbarvenou srst. Obvykle má světlejší končetiny a bílý obřitek. Samci jsou o něco větší než samice.
Za potravou se vydává zrána nebo navečer a vyhledává ji především v otevřených biomech (na polích, lukách), přes nejteplejší část dne odpočívá v pelechu ve stínu stromů. V létě se živí především trávou nebo jinými rostlinami, občas si zpestří jídelníček i lesními plody nebo žaludy, v zimě okousává větvičky jehličnatých stromů. Samice obvykle žijí spolu s mláďaty v menších skupinách, samci jsou po většinu roku samotářští, mladí pak tvoří mládenecké skupiny.
Jelenec ušatý se páří obvykle na podzim. V období páření u samců vzniká několik soubojů o samice a území. Samice je březí 190-200 dní a jedno až dvě mláďata rodí na jaře. Mláďata matku opouští na podzim, po 60-75 dnech života.
V tomto článku byl použit překlad textu z článku Mule deer na anglické Wikipedii.
Jelenec ušatý (Odocoileus hemionus) je přežvýkavec z čeledě jelenovitých (Cervidae), který žije na západě Severní Ameriky. Jedná se o největšího zástupce z jelenců. Jelenec ušatý má typické, mule podobné, velké uši, od kterých je odvozen jeho druhový název.
Der Maultierhirsch (Odocoileus hemionus) oder Großohrhirsch ist ein im Westen Nordamerikas verbreiteter Hirsch. Er ist der nächste Verwandte des Weißwedelhirsches. Es werden mehrere Unterarten unterschieden, die sich in zwei Gruppen untergliedern lassen. Jene westlich der Rocky Mountains werden meist als Schwarzwedelhirsche bezeichnet. Die Artbezeichnung Maultierhirsch bezieht sich auf die großen Ohren, die an jene von Maultieren erinnern.
Die Männchen der Maultierhirsche haben ein Widerristhöhe von einem Meter und eine Kopf-Rumpf-Länge von knapp zwei Metern. Im Durchschnitt wiegen die Männchen zwischen 79 und 91 Kilogramm, sehr kapitale Hirsche erreichen gelegentlich auch ein Gewicht von bis zu 204 Kilogramm.[1] Der schwerste, bislang gewogene Hirsch wurde 1938 in Colorado geschossen und wog 237 Kilogramm.[2] Die Weibchen wiegen rund ein Drittel weniger als die Männchen. Die Ohren erreichen eine Länge von 28 und eine Breite von 15 Zentimetern.
Bei der Nominatform ist der Schwanz weiß bis auf eine rund 5 Zentimeter lange Spitze. Bei einigen der Unterarten ist der Schwanz dagegen gänzlich schwarz. Das Sommerhaarkleid ist rötlich braun, das Winterhaarkleid, das dichte graue Wollhaare aufweist, ist dagegen graubraun.
Für den Maultierhirsch lassen sich mit Schritt, Trab und Galopp drei Gangarten unterscheiden. Das schrittweise Ziehen ist die übliche Fortbewegungsweise. Aufgeschreckte Maultierhirsche zeigen allerdings auch Prellsprünge. Dabei stoßen sie sich mit allen vier Läufen zugleich in die Höhe. Dieser Sprung, der auch für eine Reihe von Antilopen typisch ist und auch vom Damhirsch gezeigt wird, ist sehr kraftanstrengend. Er erlaubt Maultierhirschen aber, auch sehr schnell einen steilen Hang hinauf zu springen und einem Fressfeind zu entkommen. Beim Galopp erreichen Maultierhirsche eine Geschwindigkeit von knapp 60 km/h.[3]
Der Maultierhirsch lebt vor allem in den Rocky Mountains, aber auch in den Nadelwäldern von British Columbia, im Westen der Prärie und in den Halbwüsten und Wüsten der südwestlichen USA und des nordwestlichen Mexiko. In den meisten Regionen seines Verbreitungsgebietes hält sich der Maultierhirsch in bergigen Regionen auf bis Schneefall ihn zwingt, in niedrigere Höhenlagen zu ziehen. Die Herbst- und Frühjahrswanderungen dieser Hirschart können bis zu 160 Kilometer weit sein.[4] Auf Wanderungen tun sich Maultierhirsche manchmal auch zu größeren Herden zusammen.
Anders als der Weißwedelhirsch ist der Maultierhirsch kein Kulturfolger und meidet die Nähe menschlicher Siedlungen.
Der Maultierhirsch lässt sich in zwei Unterartengruppen aufteilen: einmal die der Schwarzwedelhirsche, die durch den kleinen Sitka-Schwarzwedelhirsch (O. h. sitkensis) und den Columbia-Schwarzwedelhirsch (O. h. columbianus) repräsentiert sind, und zum anderen in die eigentlichen Maultierhirsche im engeren Sinne. Zu letzteren zählen der Kalifornische Maultierhirsch (O. h. californicus), der Rocky-Mountain-Maultierhirsch (O. h. hemionus) sowie O. h. eremicus aus den Wüstengebieten des Südwestens. Schwarzwedelhirsche sind deutlich kleiner als typische Mautierhirsche. Der Kalifornische Maultierhirsch bildet in gewisser Weise eine Übergangsform zwischen dem Schwarzwedelhirsch und dem Rocky-Mountain-Maultierhirsch. Südlich des Kalifornischen Maultierhirsches leben mit O. h. fulginatus und O. h. peninsulae zwei weitere Unterarten. Letztere kommt nur im Süden der Halbinsel Niederkalifornien vor. Eine kleine Form, O. h. cerrosensis, lebt zudem auf der Insel Cedros vor der Mexikanischen Küste. Bisweilen werden die Maultierhirsche an den Osthängen der Sierra Nevada als eigene Unterart abgetrennt. Allerdings ähneln sie stark den Kalifornischen Maultierhirschen an den Westhängen des Gebirges, und somit ist ihr Status zweifelhaft.[5]

Maultierhirsche fressen überwiegend Gräser, Kräuter, Beeren, Früchte, Zweige und die Triebe von Büschen und Bäumen. Sie äsen überwiegend früh am Morgen und am späten Nachmittag. Insbesondere in den heißeren Regionen ihres Verbreitungsgebietes meiden sie Aktivitäten während der heißesten Tageszeit.[6]
Männchen und Weibchen leben in voneinander getrennten Verbänden, ältere Männchen gelegentlich auch als Einzelgänger. Grundeinheit der von Weibchen gebildeten Rudel sind Mutterfamilien. Generell lebt der Maultierhirsch in größeren Rudeln als der Weißwedelhirsch. Der Zusammenhalt dieser Gruppen ist lose, und nur zur Paarungszeit entwickelt sich eine strenge Hierarchie. Die Brunftzeit beginnt Ende Oktober, der Höhepunkt der Brunft fällt in den Winter. In der Brunftzeit versuchen die Männchen, die Führung einer Gruppe weiblicher Hirsche zu gewinnen. Dabei werden eventuell bereits anwesende jüngere und schwächere Männchen vertrieben. Die Größe des Geweihs bestimmt oftmals den Erfolg.
Die Tragezeit beträgt 210 Tage, meist werden Zwillinge geborgen. Die Kälber der Maultierhirsche haben ein geflecktes Fell. Während der ersten zwei bis drei Wochen ihres Lebens folgen sie dem Muttertier nicht, sondern liegen wartend in der Deckung, bis das Muttertier zurückkehrt, um sie zu säugen. Die Fleckzeichnung ihres Jugendkleides verlieren sie im Alter von etwa vier Monaten. Die enge Bindung an das Muttertier währt etwa ein Jahr. Gewöhnlich vertreibt das Weibchen den ihr folgenden Nachwuchs zu einem Zeitpunkt, der kurz vor der Geburt des diesjährigen Nachwuchses liegt.[7]
In den Regionen, in denen sich das Verbreitungsgebiet des Maultierhirsches mit dem des Weißwedelhirsches überlappt, kommt es gelegentlich zu Kreuzungen dieser beiden Arten. Meistens ist dabei das Muttertier eine Maultierhirschkuh. Die Männchen der Weißwedelhirsche setzen sich gegenüber den Männchen der Maultierhirsche beim Werben um ein brunftiges Weibchen durch, weil sie schneller sind als Maultierhirsche und in der Verfolgung des brünftigen Weibchens auch deutlich hartnäckiger. Die Nachkommen sind zwar fruchtbar, sie haben jedoch eine höhere Mortalitätsrate als die Nachkommen von Maultier- oder Weißwedelhirschen. Weder zeigen sie so ausgeprägte Prellsprünge wie reine Maultierhirsche noch erreichen sie die Fluchtgeschwindigkeit und Ausdauer von Weißwedelhirschen und fallen daher eher Fressfeinden zum Opfer.[8]
Der bislang nachweislich älteste Maultierhirsch war eine Hirschkuh in British-Columbien, die ein Alter von 22 Jahren erreichte.[9] Zu den Fressfeinden des Maultierhirsches zählen verwilderte Hunde, Kojoten, Wölfe, Kanadischer Luchs und Rotluchs, Puma sowie Bären. Der Puma ist vermutlich der wichtigste Fressfeind des Maultierhirsches.[10]
Von 10 Millionen Maultierhirschen, die ursprünglich in Nordamerika lebten, waren die Populationen bis 1900 durch übermäßige Bejagung auf 300.000 zurückgegangen. Durch wirksame Schutzmaßnahmen sind die Zahlen wieder auf 5 Millionen gestiegen, stagnieren aber seit den 1960er Jahren. Seit den späten 1960er Jahren ist das Vorkommen von Chronic Wasting Disease, einer ansteckenden Erkrankung des zentralen Nervensystems bei Hirschen, dokumentiert. Eine Bedrohung stellt auch die steigende Bestandszahl an Weißwedelhirschen dar. Dies geschieht zum einen durch die Einkreuzung durch Weißwedelhirsche. Weißwedelhirsche übertragen außerdem einen Parasiten auf Maultierhirsche, der diese Art stärker schwächt als den Weißwedelhirsch.[11]
Von der IUCN als bedroht geführt wird die Unterart Odocoileus hemionus cerrosensis, der Cedros-Maultierhirsch. Er ist auf die kleine mexikanische Insel Cedros vor der Küste von Baja California beschränkt. Die Population wird auf 300 Tiere geschätzt.
Der Maultierhirsch (Odocoileus hemionus) oder Großohrhirsch ist ein im Westen Nordamerikas verbreiteter Hirsch. Er ist der nächste Verwandte des Weißwedelhirsches. Es werden mehrere Unterarten unterschieden, die sich in zwei Gruppen untergliedern lassen. Jene westlich der Rocky Mountains werden meist als Schwarzwedelhirsche bezeichnet. Die Artbezeichnung Maultierhirsch bezieht sich auf die großen Ohren, die an jene von Maultieren erinnern.
Lo cèrvi emione o cèrvi mulet o encara cèrvi de coa negra (Odocoileus hemionus) es un Cervid originari dels bòsques de l'oèst de l'America del Nòrd. Se destria del cèrvi de Virgínia per sas banas pus drechas.
Lo cèrvi emione o cèrvi mulet o encara cèrvi de coa negra (Odocoileus hemionus) es un Cervid originari dels bòsques de l'oèst de l'America del Nòrd. Se destria del cèrvi de Virgínia per sas banas pus drechas.
கோவேறு கழுதை மான் (Odocoileus hemionus) என்பது வட அமெரிக்காவின் மேற்குப் பகுதியை தாயகமாகக் கொண்ட ஒரு மான் இனம் ஆகும். இவற்றின் காதுகள் கோவேறு கழுதையை ஒத்திருப்பதால் இப்பெயர் பெற்றது. இதில் கருவால் மான் உள்ளிட்ட பல்வேறு துணையினங்கள் உள்ளன.[1][3][4][5][6][7]
கோவேறு கழுதை மான் (Odocoileus hemionus) என்பது வட அமெரிக்காவின் மேற்குப் பகுதியை தாயகமாகக் கொண்ட ஒரு மான் இனம் ஆகும். இவற்றின் காதுகள் கோவேறு கழுதையை ஒத்திருப்பதால் இப்பெயர் பெற்றது. இதில் கருவால் மான் உள்ளிட்ட பல்வேறு துணையினங்கள் உள்ளன.
The mule deer (Odocoileus hemionus) is a deer indigenous to western North America; it is named for its ears, which are large like those of the mule. Two subspecies of mule deer are grouped into the black-tailed deer.[1][5][6][7][8][9]
Unlike the related white-tailed deer (Odocoileus virginianus), which is found throughout most of North America east of the Rocky Mountains and in the valleys of the Rocky Mountains from Idaho and Wyoming northward, mule deer are only found on the western Great Plains, in the Rocky Mountains, in the southwest United States, and on the west coast of North America. Mule deer have also been introduced to Argentina and Kauai, Hawaii.[5]
Mule deer can be divided into two main groups: the mule deer (sensu stricto) and the black-tailed deer. The first group includes all subspecies, except O. h. columbianus and O. h. sitkensis, which are in the black-tailed deer group.[5] The two main groups have been treated as separate species, but they hybridize, and virtually all recent authorities treat the mule deer and black-tailed deer as conspecific.[1][5][6][7][9][10] Mule deer apparently evolved from the black-tailed deer.[9] Despite this, the mtDNA of the white-tailed deer and mule deer is similar, but differs from that of the black-tailed deer.[9] This may be the result of introgression, although hybrids between the mule deer and white-tailed deer are rare in the wild (apparently more common locally in West Texas), and the hybrid survival rate is low even in captivity.[8][9] Many claims of observations of wild hybrids are not legitimate, as identification based on external features is complicated.[8]
Some authorities have recognized O. h. crooki as a senior synonym of O. h. eremicus, but the type specimen of the former is a hybrid between the mule deer and white-tailed deer, so the name O. h. crooki is invalid.[5][11] Additionally, the validity of O. h. inyoensis has been questioned, and the two insular O. h. cerrosensis and O. h. sheldoni may be synonyms of O. h. eremicus or O. h. peninsulae.[10]
The 10 valid subspecies, based on the third edition of Mammal Species of the World, are:[5]



The most noticeable differences between white-tailed and mule deer are ear size, tail color, and antler configuration. In many cases, body size is also a key difference. The mule deer's tail is black-tipped, whereas the white-tailed deer's is not. Mule deer antlers are bifurcated; they "fork" as they grow, rather than branching from a single main beam, as is the case with white-tails.
Each spring, a buck's antlers start to regrow almost immediately after the old antlers are shed. Shedding typically takes place in mid-February, with variations occurring by locale.
Although capable of running, mule deer are often seen stotting (also called pronking), with all four feet coming down together.
The mule deer is the larger of the three Odocoileus species on average, with a height of 80–106 cm (31–42 in) at the shoulders and a nose-to-tail length ranging from 1.2 to 2.1 m (3.9 to 6.9 ft). Of this, the tail may comprise 11.6 to 23 cm (4.6 to 9.1 in). Adult bucks normally weigh 55–150 kg (121–331 lb), averaging around 92 kg (203 lb), although trophy specimens may weigh up to 210 kg (460 lb). Does (female deer) are smaller and typically weigh from 43 to 90 kg (95 to 198 lb), with an average of around 68 kg (150 lb).[12][13][14][15]
Unlike the white-tailed, the mule deer does not generally show marked size variation across its range, although environmental conditions can cause considerable weight fluctuations in any given population. An exception to this is the Sitka deer subspecies (O. h. sitkensis). This race is markedly smaller than other mule deer, with an average weight of 54.5 kg (120 lb) and 36 kg (79 lb) in males and females, respectively.[16]
In addition to movements related to available shelter and food, the breeding cycle is important in understanding deer behavior. The "rut" or mating season usually begins in the fall as does go into estrus for a period of a few days and males become more aggressive, competing for mates. Does may mate with more than one buck and go back into estrus within a month if they did not become pregnant. The gestation period is about 190–200 days, with fawns born in the spring.[17] The survival rate of the fawns during labor is about 50%.[18] Fawns stay with their mothers during the summer and are weaned in the fall after about 60–75 days. Mule deer females usually give birth to two fawns, although if it is their first time having a fawn, they often have just one.[17]
A buck's antlers fall off during the winter, then grow again in preparation for the next season's rut. The annual cycle of antler growth is regulated by changes in the length of the day.[17][19]
The size of mule deer groups follows a marked seasonal pattern. Groups are smallest during fawning season (June and July in Saskatchewan and Alberta) and largest in early gestation (winter; February and March in Saskatchewan and Alberta).[19]
Besides humans, the three leading predators of mule deer are coyotes, wolves, and cougars. Bobcats, Canada lynx, wolverines, American black bears, and grizzly bears may prey upon adult deer, but most often only attack fawns or infirm specimens, or eat a deer after it has died naturally. Bears and smaller-sized carnivores are typically opportunistic feeders, and pose little threat to a strong, healthy mule deer.[13]
In 99 studies of mule deer diets, some 788 species of plants were eaten by mule deer, and their diets vary greatly depending on the season, geographic region, year, and elevation.[20] The studies[21] gave these data for Rocky Mountain mule deer diets:[22]
The diets of mule deer are very similar to those of white-tailed deer in areas where they coexist.[23][20] Mule deer are intermediate feeders rather than pure browsers or grazers; they predominantly browse, but also eat forb vegetation, small amounts of grass, and where available, tree or shrub fruits such as beans, pods, nuts (including acorns), and berries.[20][22]
Mule deer readily adapt to agricultural products and landscape plantings.[24][25] In the Sierra Nevada range, mule deer depend on the lichen Bryoria fremontii as a winter food source.[26]
The most common plant species consumed by mule deer are:
Mule deer have also been known to eat ricegrass, gramagrass, and needlegrass, as well as bearberry, bitter cherry, black oak, California buckeye, ceanothus, cedar, cliffrose, cottonwood, creek dogwood, creeping barberry, dogwood, Douglas fir, elderberry, Fendlera species, goldeneye, holly-leaf buckthorn, jack pine, knotweed, Kohleria species, manzanita, mesquite, pine, rabbitbrush, ragweed, redberry, scrub oak, serviceberry (including Pacific serviceberry), Sierra juniper, silktassel, snowberry, stonecrop, sunflower, tesota, thimbleberry, turbinella oak, velvet elder, western chokecherry, wild cherry, and wild oats.[27] Where available, mule deer also eat a variety of wild mushrooms, which are most abundant in late summer and fall in the southern Rocky Mountains; mushrooms provide moisture, protein, phosphorus, and potassium.[20][27]
Humans sometimes engage in supplemental feeding efforts in severe winters in an attempt to avoid mule deer starvation. Wildlife agencies discourage such efforts, which cause harm to mule deer populations by spreading disease (such as tuberculosis and chronic wasting disease) when deer congregate for feed, disrupting migratory patterns, causing overpopulation of local mule deer populations, and cause habitat destruction overbrowsing of shrubs and forbs. Supplemental feeding efforts might be appropriate when carefully conducted under limited circumstances, but to be successful, the feeding must begin early in the severe winter (before poor range conditions and severe weather cause malnourishment or starvation) and must be continued until range conditions can support the herd.[28]
Mule deer are variably gregarious, with a large proportion of solitary individuals (35 to 64%) and small groups (groups with ≤5 deer, 50 to 78%).[29][30] Reported mean group size measurements are three to five and typical group size (i.e. crowding) is about seven.[19][31]
Mule deer foraging on a late winter morning at Okanagan Mountain Provincial Park
Male Rocky Mountain mule deer (O. h. hemionus) in Zion National Park
Male O. h. hemionus near Leavenworth, Washington
Female Columbian black-tailed deer (O. h. columbianus) in Olympic National Park
Mule deer are ruminants, meaning they employ a nutrient acquisition strategy of fermenting plant material before digesting it. Deer consuming high-fiber, low-starch diets require less food than those consuming high-starch, low-fiber diets. Rumination time also increases when deer consume high-fiber, low-starch diets, which allows for increased nutrient acquisition due to greater length of fermentation.[32] Because some of the subspecies of mule deer are migratory, they encounter variable habitats and forage quality throughout the year.[33] Forages consumed in the summer are higher in digestible components (i.e. proteins, starches, sugars, and hemicellulose) than those consumed in the winter. The average gross energy content of the consumed forage material is 4.5 kcal/g.[34]
Due to fluctuations in forage quality and availability, mule deer fat storage varies throughout the year, with the most fat stored in October, which is depleted throughout the winter to the lowest levels of fat storage in March. Changes in hormone levels are indications of physiological adjustments to the changes in the habitat. Total body fat is a measure of the individual's energy reserves, while thyroid hormone concentrations are a metric to determine the deer's ability to use the fat reserves. Triiodothyronine (T3) hormone is directly involved with basal metabolic rate and thermoregulation.[35]

Mule deer migrate from low elevation winter ranges to high elevation summer ranges.[36] Although not all individuals in populations migrate, some will travel long distances between summer and winter ranges.[37] Researchers discovered the longest mule deer migration in Wyoming spanning 150 miles from winter to summer range[36] Multiple US states track mule deer migrations.[38][39][40][41]
Mule deer migrate in fall to avoid harsh winter conditions like deep snow that covers up food resources, and in spring follow the emergence of new growth northwards.[42][43] There is evidence to suggest that mule deer migrate based on cognitive memory, meaning they use the same path year after year even if the availability of resources has changed. This contradicts the idea that animals will go to the areas with the best available resources, which makes migratory paths crucial for survival.[43]
There are many risks that mule deer face during migration including climate change and human disturbance. Climate change impacts on seasonal growth patterns constitute a risk for migrating mule deer by invalidating historic or learned migration paths.[44][45]
Human activities such as natural resource extraction, highways, fencing, and urban development all have an impact on mule deer populations and migrations through habitat degradation and fragmentation.[46][47][48][49] Natural gas extraction has been found to have varying negative effects on mule deer behavior and can even cause them to avoid areas they use to migrate.[46] Highways not only cause injury and death to mule deer, but they can also serve as a barrier to migration.[50] As traffic volumes increase, the more mule deer tend to avoid those areas and abandon their typical migration routes. It has also been found that fencing can alter deer behavior, acting as a barrier, and potentially changing mule deer migration patterns.[51] In addition, urban development has replaced mule deer habitat with subdivisions, and human activity has increased. As a result of this, researchers have seen a decline in mule deer populations. This is especially prominent in Colorado where the population has grown by over 2.2 million since 1980.[49]
Protecting migrations corridors is essential to maintain healthy mule deer populations. One thing everyone can do is help slow the increase in climate change by using greener energy sources and reducing the amount of waste in our households.[52] In addition, managers and researchers can assess the risks listed above and take the proper steps to mitigate any adverse impacts those risk have on mule deer populations. Not only will populations benefit from these efforts but so will many other wildlife species.[53]
One way to help protect deer from getting hit on roadways is to install high fence wildlife fencing with escape routes.[54] This helps keep deer off the road, preventing vehicle collisions and allowing animals that are trapped between the road and the fence a way to escape to safety.[54] However, to maintain migration routes that cross busy highways, managers have also implemented natural, vegetated, overpasses and underpasses to allow animals, like mule deer, to migrate and move safely across highways.[55]
Approaches to mitigating the impact of drilling and mining operations include regulating the time of year when active drilling and heavy traffic to sites are taking place, and using well-informed planning to protect critical deer habitat and using barriers to mitigate the activity, noise, light at the extraction sites.[56]
The increase in urbanization has impacted mule deer migrations and there is evidence to show it also disrupts gene flow among mule deer populations.[57] One clear option is to not build houses in critical mule deer habitat; however, build near mule deer habitat has resulted in some deer becoming accustom to humans and the resources, such as food and water.[58] Rather than migrate through urban areas some deer tend to stay close to those urban developments, potentially for resources and to avoid the obstacles in urban areas.[59] Suggested measures by property owners to protect mule deer genetic diversity and migration paths include planting deer-resistant plants, placing scare devices such as noise-makers, and desisting from feeding deer.[58]
Wildlife officials in Utah announced that a November–December 2021 field study had detected the first case of SARS-CoV-2 in mule deer. Several deer possessed apparent SARS-CoV-2 antibodies, however a female deer in Morgan County had an active Delta variant infection.[60] White-tailed deer, which are able to hybridize with mule deer and which have shown high rates of SARS-CoV-2 infection, have migrated into Morgan County and other traditional mule deer habitats since at least the early 2000s.[61][62]
The mule deer (Odocoileus hemionus) is a deer indigenous to western North America; it is named for its ears, which are large like those of the mule. Two subspecies of mule deer are grouped into the black-tailed deer.
Unlike the related white-tailed deer (Odocoileus virginianus), which is found throughout most of North America east of the Rocky Mountains and in the valleys of the Rocky Mountains from Idaho and Wyoming northward, mule deer are only found on the western Great Plains, in the Rocky Mountains, in the southwest United States, and on the west coast of North America. Mule deer have also been introduced to Argentina and Kauai, Hawaii.
La Hemiona cervo (Odocoileus hemionus) estas cervo kiu estas indiĝena de okcidenta Nordameriko. Ĝi ricevas ankaŭ la komunan nomon de Mula cervo pro siaj grandaj oreloj kvazaŭ de mulo. Oni supozas, ke estas kelkaj subspecioj, inklude tiun de la Nigravosta cervo.[1][2][3][4][5][6] Malkiel sia kuzo, la Blankavosta cervo, la Mulaj cervoj estas ĝenerale pli asociaj kun la okcidento de la rivero Misuro, aŭ pli specife, kun la regiono de Rokmontaro de Nordameriko. Tiu nearktisa cervo estis ankaŭ enmetita en Kauai (Havajo) kaj Argentino.[2] La plej rimarkindaj diferencoj inter la Blankavosta cervo kaj la Hemiona cervo estas la grando de ties oreloj, la koloro de ties vostoj, kaj la formo de ties kornoj. En multaj kazoj, ankaŭ la korpogrando estas ŝlosila diferenco.
La Hemiona cervo (Odocoileus hemionus) estas cervo kiu estas indiĝena de okcidenta Nordameriko. Ĝi ricevas ankaŭ la komunan nomon de Mula cervo pro siaj grandaj oreloj kvazaŭ de mulo. Oni supozas, ke estas kelkaj subspecioj, inklude tiun de la Nigravosta cervo. Malkiel sia kuzo, la Blankavosta cervo, la Mulaj cervoj estas ĝenerale pli asociaj kun la okcidento de la rivero Misuro, aŭ pli specife, kun la regiono de Rokmontaro de Nordameriko. Tiu nearktisa cervo estis ankaŭ enmetita en Kauai (Havajo) kaj Argentino. La plej rimarkindaj diferencoj inter la Blankavosta cervo kaj la Hemiona cervo estas la grando de ties oreloj, la koloro de ties vostoj, kaj la formo de ties kornoj. En multaj kazoj, ankaŭ la korpogrando estas ŝlosila diferenco.
El venado burra o ciervo mulo (Odocoileus hemionus) es una especie de mamífero artiodáctilo de la familia Cervidae.[2] Es propio de América del Norte, y se encuentra en Canadá, Estados Unidos y México, así como en Argentina, donde ha sido introducido.[1] Debe su nombre a sus largas orejas que parecidas a las de una mula. Su pariente más próximo es el ciervo de cola blanca (Odocoileus virginianus). Las dos especies comparten a menudo hábitats naturales, y se pueden confundir uno con el otro. Las diferencias más específicas entre los dos son el color de sus colas, y sus cornamentas. La cola del ciervo mulo es negra inclinada. Las cornamentas de los ciervos mulo se bifurcan mientras que crecen ampliándose más bien hacia adelante. Cada año las cornamentas del ciervo mulo comienzan a crecer partir de mediados de enero hasta mediados de abril. Las cornamentas del venado mulo también tienden a crecer algo más grandes que las del venado de cola blanca, particularmente en climas fríos, y tienen orejas algo más prominentes.
Se reconocen las siguientes subespecies:[2]
Es de mayor tamaño y más robusto que el venado cola blanca. Las astas se ramifican en forma dicotómica, en contraste con el venado cola blanca en el son digitiformes. La cola es poco poblada en pelo, de un color homogéneo amarillento con la punta rematada con pelos negros. Las orejas son muy grandes. Los machos adultos presentan una coloración rojiza obscura o casi negra del testuz. Tienen un tamaño similar al venado de cola blanca y un largo de cuerpo similar.[3]
Es un venado ramoneador y pastador, y su dieta varia espacial y temporalmente estando constituida por hojas tiernas, yemas y frutos de árboles y arbustos, así como de diversas hierbas y pasto verde. Aunque es una especie adaptada a ambientes xéricos, requiere de agua la mayor parte del año y las poblaciones están influenciadas por este factor.[4] Las épocas de apareamiento y reproducción varían un poco dependiendo la latitud, pero en general el apareamiento sucede entre diciembre y febrero; mientras que los nacimientos suceden entre agosto y septiembre después de 208 días de gestación en promedio. Dependiendo la calidad del hábitat y posiblemente de la edad de la hembra, pueden nacer hasta dos crías. Es una especie relativamente gregaria en comparación al venado cola blanca. Las hembras con crías en desarrollo y las del año anterior forman unidades sociales de dos a ocho individuos. Los machos juveniles se asocian en grupos de cuatro a diez individuos.[5] Los machos son solitarios. Su sistema de apareamiento es la poligamia. Las áreas de actividad varían entre 14 y 45 km² para los machos, y de 2 a 18 km² para las hembras.
No es una especie en peligro pero a nivel subespecífico se considera que O. h. cerrocensis, O. h. fulginatus, O. h. penninsulae y O. h. sheldoni se encuentran en las listas de especies en peligro de extinción.
El venado burra o ciervo mulo (Odocoileus hemionus) es una especie de mamífero artiodáctilo de la familia Cervidae. Es propio de América del Norte, y se encuentra en Canadá, Estados Unidos y México, así como en Argentina, donde ha sido introducido. Debe su nombre a sus largas orejas que parecidas a las de una mula. Su pariente más próximo es el ciervo de cola blanca (Odocoileus virginianus). Las dos especies comparten a menudo hábitats naturales, y se pueden confundir uno con el otro. Las diferencias más específicas entre los dos son el color de sus colas, y sus cornamentas. La cola del ciervo mulo es negra inclinada. Las cornamentas de los ciervos mulo se bifurcan mientras que crecen ampliándose más bien hacia adelante. Cada año las cornamentas del ciervo mulo comienzan a crecer partir de mediados de enero hasta mediados de abril. Las cornamentas del venado mulo también tienden a crecer algo más grandes que las del venado de cola blanca, particularmente en climas fríos, y tienen orejas algo más prominentes.
Odocoileus hemionus Odocoileus generoko animalia da. Artiodaktiloen barruko Capreolinae azpifamilia eta Cervidae familian sailkatuta dago
Odocoileus hemionus Odocoileus generoko animalia da. Artiodaktiloen barruko Capreolinae azpifamilia eta Cervidae familian sailkatuta dago
Muulipeura eli mustahäntäpeura eli isokorvapeura (Odocoileus hemionus) on Pohjois-Amerikan länsiosissa elävä peuralaji. Se on samoin pohjoisamerikkalaisen valkohäntäpeuran lähin sukulainen. Muulipeura ja valkohäntäpeura kykenevät lisääntymään keskenään. Näiden kahden lajin välisiä risteymiä on joskus erehdytty pitämään omana lajinaan.[2] Nisäkäsnimistötoimikunnan ehdotus lajin uudeksi suomenkieliseksi nimeksi on mustahäntäkauris.[3]
Muulipeura painaa 43-150 kg, keskimäärin noin 95 kg. Se on saanut nimensä suurista, muulin korvia muistuttavista korvistaan, sekä mustasta hännän päästä, joka erottaa sen valkohäntäpeurasta. Toisin kuin valkohäntäpeuralla, muulipeuran sarvet haarautuvat dikotomisesti, aina kahteen jokseenkin yhtä vahvaan haaraan. Vain uroksilla (hirvailla) on sarvet. Se kasvattaa uudet sarvet joka vuosi.
Muulipeuroja elää koko Pohjois-Amerikan länsiosassa, Kaakkois-Alaskasta Kalifornian niemimaalle ja Luoteis-Meksikoon. Kanadassa ja Yhdysvalloissa levinneisyys ulottuu länsirannikolta Kalliovuorten yli läntiselle tasankoalueelle Saskatchewaniin, Nebraskaan, Kansasiin ja Teksasin länsiosaan asti. Argentiinassa ja Havaijin Kauain saarella muulipeura on ihmisen istuttama vieraslaji. Laji on elinympäristönsä suhteen hyvin joustava ja monin paikoin runsaslukuinen. Se elää niin metsissä, preerialla, aavikoilla kuin vuoristoissakin.[1][4][2]
Muulipeurasta on erotettu useita maantieteellisiä alalajeja, joiden tarkasta lukumäärästä ei olla aivan yksimielisiä[1][2]. Näistä länsirannikon Odocoileus hemionus columbianus-alalajia kutsutaan yleisemmin 'mustahäntäpeuraksi' (engl. black-tailed deer), luoteista O. h. sitkensis-alalajia 'sitkanpeuraksi', ja muita 'muulipeuroiksi' (mule deer):
Muulipeura eli mustahäntäpeura eli isokorvapeura (Odocoileus hemionus) on Pohjois-Amerikan länsiosissa elävä peuralaji. Se on samoin pohjoisamerikkalaisen valkohäntäpeuran lähin sukulainen. Muulipeura ja valkohäntäpeura kykenevät lisääntymään keskenään. Näiden kahden lajin välisiä risteymiä on joskus erehdytty pitämään omana lajinaan. Nisäkäsnimistötoimikunnan ehdotus lajin uudeksi suomenkieliseksi nimeksi on mustahäntäkauris.
Odocoileus hemionus, communément appelé Cerf mulet[1], Cerf hémione ou Cerf à queue noire[2], est une espèce de Cervidés qui vit dans les forêts de l'Ouest de l'Amérique du Nord ; on en distingue onze sous-espèces qui occupent des zones forestières américaines et canadiennes de 23° à 60° de latitude, ce qui fait d'eux des animaux résistants à de nombreuses conditions climatiques[3]. Il se distingue du Cerf de Virginie par ses bois plus droits.
Odocoileus hemionus, communément appelé Cerf mulet, Cerf hémione ou Cerf à queue noire, est une espèce de Cervidés qui vit dans les forêts de l'Ouest de l'Amérique du Nord ; on en distingue onze sous-espèces qui occupent des zones forestières américaines et canadiennes de 23° à 60° de latitude, ce qui fait d'eux des animaux résistants à de nombreuses conditions climatiques. Il se distingue du Cerf de Virginie par ses bois plus droits.
Il cervo mulo (Odocoileus hemionus, (Rafinesque, 1817)) è un cervo che abita nella porzione occidentale del Nordamerica. Prende il nome dalla conformazione delle orecchie che somigliano a quelle di un mulo.
Suo stretto parente è il cervo dalla coda bianca. Le due specie condividono lo stesso habitat naturale e possono essere confuse. La differenza principale è il colore nero della punta della coda del cervo mulo. Un'altra sottile differenza è la crescita biforcata dei palchi del Cervo mulo mentre quelli del cervo dalla coda bianca crescono con un aggetto più marcatamente frontale. I cervi mulo tendono a essere leggermente più grandi. I suoi nemici naturali sono il lupo, il grizzly, il coyote, il puma e il baribal.
Cervo dalla coda nera di Sitka (O. h. sitkensis)
Cervo dalla coda nera (O. h. columbianus)
Cervo mulo della California (O. h. californicus)
Cervo mulo del sud (O. h. fuliginatus)
Cervo mulo della penisola (O. h. peninsulae)
Cervo mulo del deserto (O. h. eremicus)
Cervo mulo delle Montagne Rocciose (O. h. hemionus)
Alcuni studiosi considerano l'O. h. crooki come un sinonimo senior dell'O. h. eremicus, ma si tratta di un ibrido tra il cervo mulo e il cervo dalla coda bianca e l'assegnazione come O. h. crooki non è pertanto valida.[1][2] Anche la validità dell'O. h. inyoensis è stata messa in discussione, mentre le due varianti insulari O. h. cerrosensis e O. h. sheldoni potrebbero esseri sinonimi di O. h. eremicus o O. h. peninsulae.[3]
La terza edizione del Mammal Species of the World riporta le seguenti dieci sottospecie:[2]
Al pascolo nel Parco nazionale di Zion.
Femmina di cervo mulo al pascolo nello Stato dell'Alberta, Canada.
Il cervo mulo (Odocoileus hemionus, (Rafinesque, 1817)) è un cervo che abita nella porzione occidentale del Nordamerica. Prende il nome dalla conformazione delle orecchie che somigliano a quelle di un mulo.
Suo stretto parente è il cervo dalla coda bianca. Le due specie condividono lo stesso habitat naturale e possono essere confuse. La differenza principale è il colore nero della punta della coda del cervo mulo. Un'altra sottile differenza è la crescita biforcata dei palchi del Cervo mulo mentre quelli del cervo dalla coda bianca crescono con un aggetto più marcatamente frontale. I cervi mulo tendono a essere leggermente più grandi. I suoi nemici naturali sono il lupo, il grizzly, il coyote, il puma e il baribal.
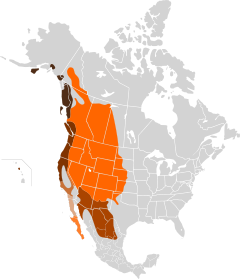
Juodauodegis elnias (lot. Odocoileus hemionus) – elninių (Cervidae) šeimos žinduolis. Paplitęs vakarinėje Šiaurės Amerikos dalyje, buvo taip pat užveistas Argentinoje.[1]
Juodauodegis elnias yra didesnis ir tvirtesnis už baltauodegį. Patinai vidutiniškai sveria 79-91 kg, nors kartais gali sverti ir iki 204 kg.[2] Patelės sveria maždaug trečdaliu mažiau. Uodegoje mažai plaukų. Ausys labai didelės, nuo 28 cm ilgio ir 15 cm pločio. Suaugę patinai turi tamsią arba beveik juodą uodegą. Vasarinis kailis yra rausvai rudas, žieminis – pilkai rudas.
Rūšis yra skirstoma į šiuos porūšius:[1]
Juodauodegis elnias (lot. Odocoileus hemionus) – elninių (Cervidae) šeimos žinduolis. Paplitęs vakarinėje Šiaurės Amerikos dalyje, buvo taip pat užveistas Argentinoje.
Melnastes briedis (Odocoileus hemionus) ir briežu dzimtas (Cervidae) pārnadzis, kura dabīgais izplatības areāls aptver Ziemeļamerikas rietumdaļu, uz rietumiem no Misūri upes, lielai populācijas daļai dzīvojot Klinšu kalnos. Melnastes briedis ir introducēts arī Kauai (vienā no Havaju salām) un Argentīnā.[1][2]
Melnastes briedis mājo Ziemeļamerikas rietumdaļā, sākot ar Aļaskas dienvidrietumiem, izplatības areālam aptverot Kanādas un ASV rietumdaļu. Rietumu robeža ir Klusā okeāna krasts, bet austrumu virzienā areāls šķērso Klinšu kalnus, sasniedzot Lielos līdzenumus, bet dienvidu virzienā ietver Kalifornijas pussalu, Sedrosas un Tiburonas salas un sasniedz Meksikas ziemeļrietumus un centrālo daļu.[2]
Melnastes briedis ir vidēja lieluma vai liels briedis. Tēviņi ir lielāki nekā mātītes. Tēviņa ķermeņa garums ir apmēram 126—168 cm, mātītēm 125—156 cm. Augstums skaustā 84—106 cm tēviņiem, 80—100 cm mātītēm. Ķermeņa svars tēviņam 45–150 kg, bet var būt arī lielāki īpatņi. Lielākais zināmais melnastes briedis ir svēris 210 kg. Svars mātītēm 43–75 kg.[3][4] Lai arī melnastes brieža pasugām raksturīgas minimālas ķermeņa lieluma atšķirības, tomēr Sitkas melnastes briedis ir mazāks par pārējiem. Tā vidējā ķermeņa masa tēviņam ir 54,5 kg, mātītei 36 kg.[5]
Abiem dzimumiem astes garums ir apmēram 11,6—23 cm. Ausis ir garas, līdz 25 cm, tās mēdz salīdzināt ar garajām mūļa ausīm. Tādēļ angļu valodā melnastes briedi sauc arī par mūļu briedi (Mule Deer).
Kažoka krāsa var būt dažāda: tumši pelēkbrūna, gaiši pelēkbrūna, gaiši brūna un pat sarkanbrūna. Ķermeņa aizmugurē uz ciskām ir liels, balts vai dzeltens laukums, purns un pakrūte baltas. Aste balta un visbiežāk ar melnu, otiņai līdzīgu astes galu, ļoti retos gadījumos ar baltu un plānu astes galu.[4][6] Dažiem īpatņiem uz kakla un muguras ir tumša garenvirziena svītra, kas beidzas pie melnā astes gala.[4] Brieža matojuma krāsa ir nemainīga visu tā mūžu. Uz sejas ir "V" veida zīme, kuras smailais gals sākas starp acīm. Zīme visskaidrāk saredzama tēviņiem. Pirmajā gadā tēviņi un mātītes attīstās vienādi, pēc tam tēviņi pāraug mātītes.[4] Melnastes briežiem nav augšējo priekšzobu, to vietā irā cietas augslejas.[7]
Melnastes briedim ir lieli, skaisti, žuburoti ragi. Ziemas beigās, agrā pavasarī, laikā no janvāra vidus līdz martam, melnastes briedis nomet ragus (atkarībā no izplatības reģionos), uzreiz pēc tam sāk augt jaunie ragi. Ragu maiņa ir cieši saistīta ar dienas un nakts garumu.[7] Visvarenākie ragi tēviņiem ir septiņu gadu vecumā, to plētums var sasniegt līdz pat 120 cm.
Salīdzinoši melnastes briedis vidēji ir lielāks nekā baltastes briedis. Vispamanāmākā atšķirība starp abām sugām ir ausu lielums. Melnastes briedim ausis ir daudz lielākas. Tā aste parasti ir viscauri balta ar melnu astes galu (reizēm uz astes virspuses ir tumša svītra kā turpinājums muguras viduslīnijai), toties baltastes briedim aste vienmēr ir balta tikai no apakšas, un astes gals ir balts. Baltastes briedis, izslienot asti vertikāli gaisā, mēdz signalizēt par briesmām. Melnastes briedis asti neceļ un nelieto signalizēšanai.[8] Arī ragu struktūra abām sugām ir atšķirīga. Melnastes briedim ragu žuburi veidojas arī uz sānu zariem, bet baltastes briedim visi žuburi ir kā sānu zari, kas atdalās no galvenā stumbra. Kopumā melnastes brieža ragi ir lielāki un žuburotāki.[8]
Melnastes briedis pamatā ir vientuļnieks, izņemot mātītes ar mazuļiem. Tomēr jauni tēviņi mēdz veidot nelielus vecpuišu barus. Tas apdzīvo dažādas biotopus, sākot ar augstiem kalniem un beidzot ar tuksnešiem un prērijām zemienēs. Melnastes briedim ir neliela teritorija, kurā tas parasti uzturas gan ziemā, gan vasarā, dienā pārvietojoties apmēram 8 km. Tādējādi teritorija lēnām var izmainīties. Reizēm melnastes brieži veic sezonālas migrācijas, pārvietojoties no augstkalniem, kuros uzturas vasarā, lejup uz zemieni, kur uzturas ziemā, cenšoties izvairīties no dziļiem sniegiem[4] Dienvidaustrumu vai dienvidrietumu nogāzēs šie dzīvnieki vasarā sastopami vietās, kas atrodas 2300 metrus virs jūras līmeņa. Baroties melnastes briedis dodas agri no rīta un vakarā.
Mātītēm veidojas ciešas savstarpējās saites pa mātes līniju, veidojoties ģimeņu klaniem. Tiem ir liela nozīmē aizsardzībā pret ienaidniekiem. Jaunie tēviņi samērā ātri pamet ģimenes grupu. Ziemā un pavasarī, kad veidojas nelieli bari, tajos valda stingra hierarhija.[4] Galvenā taktika, lai izbēgtu no ienaidniekiem, ir laicīga to pamanīšana. Līdzko briesmas ir pamanītas, dzīvnieki cenšas paslēpties garā zālē, kādā krūmu aizsegā, turpinot uzmanīgi vērot plēsēja pārvietošanos.[4] Melnastes briedim ir ne tikai lieliska dzirde, bet arī laba redze. Tēviņi var pat pamanīt citu dzīvnieku pat 600 m attālumā. Otrs veids, kā izvairīties no briesmām, ir laicīga aizbēgšana uz citu vietu, pirms plēsējs ir pietuvojies. Drošs paņēmiens, lai izvairītos no ienaidniekiem, ir uzkāpšana augstā kalnā, tādējādi plēsējam liekot pārdomāt, vai tam pietiks spēki medībām stāvajā reljefā. Bēgot melnastes briedis bieži maina virzienu, izdarot neparedzamu rāvienus vai arī meklē aizsegu, kādu šķērsli starp sevi un plēsēju. Melnastes briedis ļoti labi peld, bet pie bēgšanas to reti izmanto.[4] Melnastes brieži, lai arī spēj ātri skriet, tomēr tos ļoti bieži var redzēt lecam uz visām četrām kājām vienlaicīgi.
Melnastes briedis ir atgremotāju apakškārtas dzīvnieks, un viņu populācija tiešā nozīmē ir atkarīga no sulīgas un viegli sagremojamas barības. To diēta sastāv no koku zariem un stiebrzālēm, kas savstarpēji veido apmēram vienlīdzīgu apjomu.
Melnastes briedim ir vajadzīgs barojošs uzturs. Vasarā šis dzīvnieks labprāt apgrauž pumpurus, skujkoku vai alkšņu zarus. Melnastes briedis ēd arī sēnes, ozolzīles, lauka pupas, ogas un kauleņus, kuri ir bagātīgi ar kokšķiedrām, kas labi pārstrādājas, svaigus augļus, riekstus un ķērpjus. Ziemā, kad trūkst barības, melnastes brieži apvienojas lielākās grupās. Dzīvnieki kopīgi atrakņā sniegu, lai piekļūtu barībai. Šāda darbība ievērojami bojā zāles dzinumus. Kad melnastes briežu bars nogana vienu vietu, dzīvnieki dodas citu barošanās vietu meklējumos.[4]
Melnastes brieži ir poligāmi, un to riests sākas rudenī. Sezonas aktīvākie mēneši ir novembris un decembris. Šajā laikā tēviņi kļūst kareivīgi un agresīvi. Mātītes meklēšanās ir samērā īss brīdis, tikai dažas dienas. Pāroties gatavas mātītes tuvumā starp tēviņiem sākas savstarpējās cīņas, laužoties ar ragiem. Vājākais no sāncenšiem padodas, iekams cīņa izvēršas par bīstamu sadursmi. Dominantais tēviņš sezonā sapārojas visbiežāk.[4] Mātīte var sapāroties ar vairākiem tēviņiem, bet, ja šajās dienās tā netiek apaugļota, paiet apmēram mēnesis, līdz mātīte atkal meklējas. Mātītes parasti pārojas otrajā gadā, ļoti reti pirmajā gada.
Grūsnības periods ilgst 182-210 dienas. Jūnijā vai jūlijā dzimst mazuļi.[4] Lai arī parasti dzimst divi mazuļi, jaunām mātītēm ļoti bieži dzimst tikai viens mazulis.[7] Piedzimstot tas sver apmēram 2—5 kg, tēviņi piedzimst parasti lielāki un smagāki.[4] Barošana ar mātes pienu norit apmēram 2 mēnešus (60—75 dienas). Mazuļi spēj nostāvēt kājās un staigāt jau īsu brīdi pēc dzimšanas. Māte pirmo mēnesi mazuli slēpj garā un biezā zālē vai krūmājos, kur, pateicoties aizsargkrāsas kažociņam (rudi brūns ar baltiem raibumiem), viņš ir labi pasargāts no ienaidniekiem. Jaunie tēviņi pilnu augumu un masu sasniedz 49 mēnešu vecumā, mātītes 37 mēnešos, lai gan ķermeņi un muskuļu masa arī pēc tam turpina nobriest.[4] Melnastes briežu dzīves ilgums ir apmēram 9—11 gadi.[7]
Galvenie melnastes brieža ienaidnieki ir pelēkie vilki, pumas, koijoti, rūsganie lūši, tiņi, Amerikas melnie lāči un brūnie lāči, kā arī klinšu ērgļi.[4] Lai arī plēsēji var uzbrukt arī pieaugušiem īpatņiem, tomēr tie galvenokārt uzbrūk mazuļiem.[4]
Cilvēki vienmēr ir medījuši melnastes briežus. Mednieki apstrādā ādas un ēd gaļu. Medības populāciju nopietni neapdraud. Tā kā melnastes brieži reizēm var radīt lielus zaudējumus lauksaimniecībai, dažos reģionos tiek celti žogi, lai aizsargātu tīrumus. Savukārt citviet cilvēki savvaļā sēj labību, lai pirms medību sezonas piebarotu melnastes briežus. Vislielākos zaudējumus melnastes briežiem cilvēki tiešā veidā radīja 20. gadsimta sākumā. 1905.—1925. gadā mājdzīvnieku aizsardzības akcijas ietvaros Arizonas līdzenumā Kaibabā tika nošauti tūkstošiem vilku, pumu un koijotu. Bez šiem dabiskajiem ienaidniekiem melnastes briežu skaits strauji palielinājās: no sākotnējiem 4000 indivīdiem līdz 100 000. Neraugoties uz daudzkārtējiem brīdinājumiem, varas iestādes neko nedarīja, lai ierobežotu melnastes briežu populācijas pārlieko savairošanos. Rezultātā 60 000 briežu gāja bojā badā. Mūsdienās šīs sugas populācija atkal tiek stingri uzraudzīta un regulēta.[9]
Melnastes briežiem ir 10 pasugas:
Melnastes briedis (Odocoileus hemionus) ir briežu dzimtas (Cervidae) pārnadzis, kura dabīgais izplatības areāls aptver Ziemeļamerikas rietumdaļu, uz rietumiem no Misūri upes, lielai populācijas daļai dzīvojot Klinšu kalnos. Melnastes briedis ir introducēts arī Kauai (vienā no Havaju salām) un Argentīnā.
Het muildierhert (Odocoileus hemionus) is een Noord-Amerikaans hert. Het is nauw verwant aan het witstaarthert (Odocoileus virginianus). Het muildierhert dankt zijn naam aan de grote oren, die doen denken aan die van een muildier. Ook het muildierhert kan zijn oren onafhankelijk van elkaar bewegen. Het muildierhert heeft tien ondersoorten, waaronder het sitkahert.
Het muildierhert heeft een bruine vacht. Zomers is deze meer rossig tot gelig bruin, 's winters is de vacht grijziger van kleur. De buik is roomwit van kleur, de binnenzijde van de poten, de keelvlek en het binnenste van de oren zijn wit. De staart is aan de bovenzijde wit met een zwart puntje. Bij herten langs de Pacifische kust is de bovenzijde geheel zwart of donkerbruin. Kalfjes hebben een gevlekte vacht.
Het muildierhert heeft een schouderhoogte van 90 tot 105 centimeter, een lichaamslengte van 116 tot 199 centimeter en een staartlengte van 11,4 tot 23 centimeter. Mannetjes zijn groter dan vrouwtjes en dragen een gewei. De hertenbok weegt 50 tot 215 kilogram, de hinde 32 tot 73 kilogram.
Het muildierhert is vooral actief in de schemering en op nachten met een heldere maan. 's Winters laat hij zich ook overdag zien. In berggebieden trekt het muildierhert in de winter naar de dalen.
Het hert eet 's zomers kruiden en bessen, en 's winters twijgen van naald- en loofbomen. Ook eet hij eikels en appels.
Het muildierhert leeft in kleine familiegroepjes, bestaande uit een vrouwtje, haar kalfjes en soms ook de eenjarige jongen van de vorige worp. 's Winters voegen deze groepen zich vaak samen in kleine gemengde groepen. Buiten de winter zijn de hinden vrij agressief tegenover andere vrouwtjes. Hertenbokken leven solitair, maar buiten de bronsttijd kunnen ze ook in groepjes van twee leven. Het woongebied van de bok is groter dan die van de hinde.
De bronsttijd loopt van de herfst tot de vroege winter. Tijdens de bronsttijd verlaten veel dieren hun gebruikelijke woongebied. Bokken zijn polygaam en proberen soms een kudde van hindes te vormen. Ook de hinde paart meestal met meerdere bokken. Meestal vechten de bokken met elkaar door middel van dreiggedrag, maar soms wordt er gevochten met de geweien, waarbij de bokken elkaars kop naar beneden proberen te drukken. Meestal ontstaan er geen verwondingen bij deze gevechten, maar soms raken de geweien verstrikt, waarbij beide bokken zullen sterven door verhongering.
In juni en augustus worden na een draagtijd van zes tot zeven maanden één à twee kalfjes geboren. Tweelingen zijn algemener bij oudere hindes, terwijl hindes die voor de eerste keer jongen slechts één kalf krijgen. Ze wegen bij de geboorte ongeveer 3,6 kilogram. De eerste maand worden de kalfjes achtergelaten terwijl de moeder gaat grazen. Ze zoekt de kalfjes regelmatig op zodat ze kunnen worden gezoogd.
De belangrijkste natuurlijke vijanden van het muildierhert zijn de poema en de wolf. Ook lynxen en beren grijpen weleens een hert, en coyotes vangen een enkele keer een kalf. Mensen jagen ook op het muildierhert, als trofee of vanwege schade aan de landbouw.
Muildierherten leven langs bosranden, in bergen en voorgebergten in gemengde wouden. Ze leven in het westen van Noord-Amerika: in zowel de Verenigde Staten, Canada als Noord-Mexico. Vooral in de Rocky Mountains zijn ze algemeen. Het hert is ingevoerd op Hawaï en in Argentinië.
Bronnen, noten en/of referentiesHet muildierhert (Odocoileus hemionus) is een Noord-Amerikaans hert. Het is nauw verwant aan het witstaarthert (Odocoileus virginianus). Het muildierhert dankt zijn naam aan de grote oren, die doen denken aan die van een muildier. Ook het muildierhert kan zijn oren onafhankelijk van elkaar bewegen. Het muildierhert heeft tien ondersoorten, waaronder het sitkahert.
Mulhjorten (Odocoileus hemionus) er et nordamerikansk medlem av hjortefamilien, og har en meget karakteristisk hvit hale med svart halespiss og et par store muldyraktige ører, som den har fått navnet sitt fra. Mulhjorten veier vanligvis 110–140 kg, og kan nå en kroppslengde på to meter.
Mulhjortens nærmeste slektning er hvithalehjorten (Odocoileus virginianus), som har mindre ører, en annen farge på halen og et gevir som luter framover (framfor rett opp) og kløyver tettere.[1]
Mulhjorten er ikke et typisk flokkdyr, og tilbringer det meste av sitt liv alene. I næringsfattige amerikanske vintere danner den ofte flokker på opptil 50 dyr, som går sammen for å lage seg beiteplasser. Dette gjør derimot stor skade på bakken, og derfor gjør bønder mye for å forhindre at mulhjorten tar seg inn på avlingene deres.
Mulhjorten har en spesiell løpemåte, og hopper høyt mens den løper. Dette har gitt den tilnavnet «den hoppende hjorten».
Mulhjortens føde består hovedsakelig av kvister og skudd fra furu, amerikaosp og ulike busker. Om vinteren går føden for det meste på gress, og den sier heller ikke nei takk til sopp og lav.
Paringstiden foregår fra slutten av september til midten av november, og det er i denne tiden at de ellers så fredelige bukkene (hanndyrene) blir aggressive og slåss om hindene (hunndyrene). En bukk kan få opptil fire hinder for seg selv.
Drektighetstiden er på 6 – 7 måneder, og mulhjorthinden føder som oftest to kalver. Disse kalvene har de karakteristiske hvite flekkene i pelsen, som gjør at de er godt skjulte blant vegetasjonen for rovdyr.
Selv om det har blitt drevet sterk jakt på mulhjorten, er den per i dag ikke utrydningstruet. Jakten, samt de naturlige fiendene dens – puma, bjørn, jerv og lignende rovdyr – er nødvendig for å holde bestanden nede.
I tidsperioden 1905–1925 ble det drevet sterk jakt på ulv, puma og prærieulv i Arizona, og uten rovdyrene eksploderte mulhjortbestanden drastisk. Dette førte til massedød som følge av matmangel, og det ble meldt om at hele 60 000 mulhjort døde i løpet av kun ett år.
Mulhjorten finnes i det vestre USA, det sørvestlige Canada og det nordlige Mexico. Den er svært tilpasningsdyktig, og av den grunn forekommer den i mange forskjellige leveområder, alt fra fjelltrakter like under tregrensen til ørkensletter.
Mulhjorten (Odocoileus hemionus) er et nordamerikansk medlem av hjortefamilien, og har en meget karakteristisk hvit hale med svart halespiss og et par store muldyraktige ører, som den har fått navnet sitt fra. Mulhjorten veier vanligvis 110–140 kg, og kan nå en kroppslengde på to meter.
Mulhjortens nærmeste slektning er hvithalehjorten (Odocoileus virginianus), som har mindre ører, en annen farge på halen og et gevir som luter framover (framfor rett opp) og kløyver tettere.
Mulak czarnoogonowy[3] (Odocoileus hemionus) – gatunek ssaka z rodziny jeleniowatych (Cervidae).
Długość ciała 1-1,9 metra, ogon 10–25 cm, masa 50–215 kg. Ubarwienie zimowe - szare, letnie rudawe.
Zamieszkuje na terenach leśnych oraz otwartych w zachodnich obszarach Ameryki Północnej. Żyje w parach lub w małych grupach.
Mulak czarnoogonowy (Odocoileus hemionus) – gatunek ssaka z rodziny jeleniowatych (Cervidae).
Długość ciała 1-1,9 metra, ogon 10–25 cm, masa 50–215 kg. Ubarwienie zimowe - szare, letnie rudawe.
Zamieszkuje na terenach leśnych oraz otwartych w zachodnich obszarach Ameryki Północnej. Żyje w parach lub w małych grupach.
O veado-mula (Odocoileus hemionus) é um veado norte-americano.
Apresenta orelhas quase tão longas quanto sua cabeça, grandes olhos que detectam qualquer movimento fora do vulgar, os machos têm chifres que se desenvolvem até que atinjam os quatro ou cinco anos de idade, usufrui de um pêlo denso acastanhado, que se torna menos denso e um pouco mais avermelhado no Verão. Têm fortes músculos nas pernas para correr e saltar. Os machos de veado-mula são maiores e mais pesados que as fêmeas.
Este veado prefere viver em áreas onde exista vegetação abundante, onde possa se esconder de seus predadores. Enquanto come, o veado mantém-se alerta para o perigo, com os seus olhos bem abertos e as suas orelhas bem espetadas. Come uma grande variedade de ervas, dado que a sua dieta contém poucos alimentos ricos em energia, este animal de pasto pode ter de procurar todo o dia para conseguir alimento suficiente, especialmente durante o Inverno.
Apesar de alguns machos serem solitários, a maioria dos veados-mula vivem em pequenos grupos. Vários grupos podem juntar-se no Inverno para protecção contra predadores. A fêmea dá à luz sete meses depois de ter acasalado. Uma cria mama durante quatro meses mas também come alimentos sólidos. Os jovens machos deixam a progenitora ao fim de um ano, mas as fêmeas podem ficar com ela durante anos.
O veado-mula encontra-se em todo o Oeste da América do Norte, desde o Sul de Yukon, no Alasca, até a Baixa Califórnia e Norte do México. Pode ser encontrado em diversos tipos de hábitats, como em montanhas, semi-desertos, áreas costeiras, zonas húmidas e pantanosas, pradarias e florestas temperadas e conífera.
O veado-mula (Odocoileus hemionus) é um veado norte-americano.
Svartsvanshjort eller åsnehjort (Odocoileus hemionus) är en art i familjen hjortdjur som förekommer i Nordamerikas västra delar. Den är närmast släkt med vitsvanshjorten.
Allmänt liknar arten vitsvanshjorten men svansens färg är annorlunda. Svansfärgen är beroende på underart helt svart eller på bägge sidor vit med svart spets. Öronen är betydligt större och även hanarnas horn har en annan form än hos vitsvanshjorten. Hos unga hanar finns enkla stänger, som hos rådjuret, och hos äldre hanar växer flera förgreningar upp till 50 taggar. Svartsvanshjortar har en mankhöjd av cirka en meter och en vikt mellan 75 (honor) och 150 (hanar) kilogram.
Svartsvanshjortens utbredningsområde ligger huvudsakligen i Klippiga bergen men även i barrskogar av British Columbia, i västra delar av prärien samt i öken och halvöken av sydvästra USA och nordvästra Mexiko. I motsats till vitsvanshjorten är djuret ingen kulturföljare och undviker människans boplatser.
Hanar och honor lever i skilda grupper och gamla hanar ibland ensamma. Gruppens sammansättning är inte fastlagd, bara under parningstiden uppstår en fast hierarki. Under vandringar bildar svartsvanshjortar ibland större hjordar. Dessa vandringar förekommer oftast i bergsområden, för att undkomma kylan i höga regioner, och i öken, för att hitta vattenkällor.
Vid parningstiden försöker hanarna få kontroll över en grupp honor. Unga och svaga hanar fördrivs från gruppen. Oftast bestämmer hornens storlek vem som är starkast.
Ursprungligen fanns omkring 10 miljoner svartsvanshjortar i Nordamerika men efter en tid med intensiv jakt minskade beståndet till 300 000 individer året 1900. Efter inrättning av skyddsåtgärder ökade populationen till 5 miljoner exemplar men stagnerade efter 1960-talet.
IUCN listar en underart, Odocoileus hemionus cerrosensis, som hotad. Den lever på den mexikanska ön Cedros som ligger vid Baja Californias kustlinje. Populationen av underarten uppskattas till 300 individer.
Svartsvanshjort eller åsnehjort (Odocoileus hemionus) är en art i familjen hjortdjur som förekommer i Nordamerikas västra delar. Den är närmast släkt med vitsvanshjorten.
 Bilimsel sınıflandırma Âlem: Animalia Şube: Chordata Sınıf: Mammalia Takım: Artiodactyla Alt takım: Ruminantia Familya: Cervidae
Bilimsel sınıflandırma Âlem: Animalia Şube: Chordata Sınıf: Mammalia Takım: Artiodactyla Alt takım: Ruminantia Familya: CervidaeKatır geyiği (Odocoileus hemionus) geyikgiller (Cervidae) familyasından bir geyik türüdür. Kuzey Amerika'nın batı yarısında yaşar. Adını büyük kulaklarının katır kulaklarına benzemesinden alır. En yakın akrabası, aynı bölgede yaşadığı kara kuruklu geyiktir. Sıklıkla birbirleriyle karıştırılırlar. İki geyik türü arasında en dikkat çekici ayrım kuyruklarının rengi ve boynuzlarıdır. Katır geyiğinin kuyruğunun yalnızca ucu siyahtır. Katır geyiğinin boynuzları büyüdükçe ileri doğru genişleyeceğine çatallaşır. Her yıl erkek geyiğin boynuzları ocak ortasından nisan ortasına kadar çiftleşme mevsimi sonrasında dökülür ve ilkbaharda tekrar çıkmaya başlar. Katır geyiğinin erkek bireyleri özellikle soğuk iklimlerde ak kuyruklu geyiklerin erkek bireylerinden biraz daha büyük olur. Kulakları da biraz daha belirgindir. Katır geyiği koşmak yerine zıplayıp dört ayağının üstüne aynı anda düştüğü adımlarla hareket eder.
Katır geyiği (Odocoileus hemionus) geyikgiller (Cervidae) familyasından bir geyik türüdür. Kuzey Amerika'nın batı yarısında yaşar. Adını büyük kulaklarının katır kulaklarına benzemesinden alır. En yakın akrabası, aynı bölgede yaşadığı kara kuruklu geyiktir. Sıklıkla birbirleriyle karıştırılırlar. İki geyik türü arasında en dikkat çekici ayrım kuyruklarının rengi ve boynuzlarıdır. Katır geyiğinin kuyruğunun yalnızca ucu siyahtır. Katır geyiğinin boynuzları büyüdükçe ileri doğru genişleyeceğine çatallaşır. Her yıl erkek geyiğin boynuzları ocak ortasından nisan ortasına kadar çiftleşme mevsimi sonrasında dökülür ve ilkbaharda tekrar çıkmaya başlar. Katır geyiğinin erkek bireyleri özellikle soğuk iklimlerde ak kuyruklu geyiklerin erkek bireylerinden biraz daha büyük olur. Kulakları da biraz daha belirgindir. Katır geyiği koşmak yerine zıplayıp dört ayağının üstüne aynı anda düştüğü adımlarla hareket eder.
Hươu la (tiếng Anh: Mule deer, danh pháp hai phần: Odocoileus hemionus), là một loài hươu thuộc chi Odocoileus, họ Cervidae, phân họ Capreolinae, bộ Artiodactyla.
Đây là loài hươu bản địa tại phía tây Bắc Mỹ. Được đặt tên theo hình dạng lỗ tai, lớn giống như con la. Được cho là có vài phân loài, bao gồm cả hươu đuôi đen.[1][3][4][5][6][7]
Không giống như họ hàng hươu đuôi trắng (Odocoileus virginianus), hươu la gắn liền với vùng đất bờ tây sông Missouri, đặc biệt tại dãy núi Rocky của Bắc Mỹ. Hươu la cũng được du nhập đến Argentina.[3]
Sự khác biệt đáng chú ý nhất giữa hươu đuôi trắng và hươu la là kích thước đôi lỗ tai của chúng, màu sắc đuôi, và hình dạng sừng gạc. Trong nhiều trường hợp, kích thước cơ thể cũng là một sự khác biệt quan trọng. Đuôi hươu la có ngọn màu đen, trong khi hươu đuôi trắng không có. Gạc hươu la chẻ đôi; chúng "chẻ ba" khi hươu phát triển, chứ không phải phân nhánh từ một trục chính đơn nhất, như trường hợp hươu đuôi trắng. Mỗi mùa xuân, gạc hươu đực bắt đầu mọc trở lại gần như ngay lập tức sau khi gạc cũ gãy đi. Sự gãy thường diễn ra vào giữa tháng hai, với biến thể diễn ra bởi nơi xảy ra. Mặc dù có khả năng chạy, hươu la thường thấy động tác stotting (1 động tác nhảy vọt lên khi gặp nguy hiểm) (còn gọi là pronking), với cả bốn bàn chân đi xuống với nhau. Hươu đuôi đen cũng đã được du nhập đến Kauai, Hawaii.
Hươu la là loài lớn trong 2 loài thuộc chi Odocoileus trên trung bình, với chiều cao khoảng 80–106 cm (31–42 in) tại bờ vai và chiều dài từ mũi đến đuôi khoảng từ 1,2 đến 2,1 m (3,9 đến 6,9 ft). Trong số này, đuôi có thể bao gồm 11,6 đến 23 cm (4,6 đến 9,1 in). Hươu đực trưởng thành (male deer) thường nặng 55–150 kg (121–331 lb), trung bình khoảng 92 kg (203 lb), mặc dù mẫu vật trưng bày có thể nặng lên đến 210 kg (460 lb). Hươu cái trưởng thành (female deer) khá nhỏ và thường nặng từ 43 đến 90 kg (95 đến 198 lb), với mức trung bình khoảng 68 kg (150 lb).[8][9][10][11] Không giống hươu đuôi trắng, hươu la thường không cho thấy sự thay đổi kích thước đáng kể dọc theo phạm vi loài, mặc dù điều kiện môi trường có thể gây ra biến động trọng lượng đáng kể trong bất kỳ quần thể nhất định. Một ngoại lệ theo đấy, phân loài hươu đuôi đen Sitka (O. h. sitkensis). Phân loài này nhỏ hơn đáng kể so với các phân loài khác của hươu la, với trọng lượng trung bình khoảng 54,5 kg (120 lb) và 36 kg (79 lb) ở hươu đực và hươu cái, tương ứng.[12]
Trong điều kiện vận động liên quan đến chỗ ở có sẵn và thức ăn, chu kỳ sinh sản rất quan trọng trong hành vi hiểu biết của hươu. Thời kỳ "động dục ở động vật đực" hoặc mùa giao phối thường bắt đầu vào mùa thu cũng như hươu cái vào trong thời kỳ động dục ở động vật cái trong thời gian một vài ngày và hươu đực trở nên hung hăng hơn, cạnh tranh bạn tình. Hươu cái có thể giao phối với nhiều hơn một hươu đực và quay trở lại động dục trong vòng một tháng, nếu chúng không lắng dịu. Thai kỳ khoảng 190-200 ngày, hươu non sinh ra vào mùa xuân, ở với hươu mẹ trong suốt mùa hè và được cai sữa vào mùa thu sau khoảng 60-75 ngày. Hươu la cái thường sinh hai hươu con, mặc dù nếu đó là lần đầu tiên hươu sinh một con non, thường chỉ có một.[13] Gạc hươu đực gãy trong mùa đông, tăng trưởng trở lại để chuẩn bị cho động dục mùa tới. Chu kỳ tăng trưởng hàng năm của gạc hươu được quy định bởi những thay đổi trong chiều dài của ngày.[13] Để biết thêm thông tin xem bài viết chính về hươu nai.
Bên cạnh con người, ba loài săn mồi hàng đầu của hươu la là sói đồng cỏ, sói xám, và báo sư tử. Linh miêu đuôi cộc, chồn sói, gấu đen Bắc Mỹ và gấu nâu có thể săn hươu trưởng thành, nhưng thường chỉ tấn công hươu non, mẫu vật ốm yếu hoặc ăn xác hươu sau khi đã chết tự nhiên. Gấu và các loài có kích thước nhỏ hơn thường kiếm ăn cơ hội, và ít đe dọa đến một con hươu la mạnh mẽ, khỏe mạnh.[9]
Kufleld et al. (1973) đã phân tích 99 nghiên cứu về chế độ ăn của hươu la và thấy rằng một số lượng 788 loài thực vật đã được hươu la ăn, và chế độ ăn của hươu la thay đổi rất nhiều tùy thuộc vào mùa, khu vực địa lý, năm, và độ cao so với mực nước biển.[14] Kufeld, et al. (1973) đã đưa ra các số liệu sau đây về chế độ ăn của hươu la ghi nhận tại dãy núi Rocky:[15]
Cây bụi & Cây xanh Thảo mộc Cỏ thường & cỏ yêu thích Mùa đông 74% 15% 11% (biến đổi 0-53%) Mùa xuân 49% 25% 26% (biến đổi 4-64%) Mùa hè 49% 46% (biến đổi 3-77%) 3% (biến đổi 0-22%) Mùa thu 60% 30% (biến đổi 2-78%) 9% (biến đổi 0-24%)Anthony & Smith (1977) phát hiện ra rằng chế độ ăn của hươu la rất tương tự như của hươu đuôi trắng trong khu vực mà chúng cùng tồn tại.[14] Hươu la kiếm ăn trung gian chứ không phải gặm chồi non tinh khiết hoặc gặm cỏ; phần lớn là chồi non, nhưng cũng ăn thảm thực vật thảo mộc, một lượng nhỏ cỏ, hoặc nếu có thể là quả của cây xanh hoặc cây bụi như cây đậu, vỏ đậu, hạt cứng (bao gồm quả đấu, đó là hạt sồi), và quả mọng.[14][15]
Hươu la dễ dàng thích ứng với sản phẩm nông nghiệp và cây trồng cảnh quan.[16][17] Tại dãy núi Sierra Nevada, hươu la phụ thuộc vào địa y wila như 1 nguồn thức ăn mùa đông.[18]:2:4
Những loài thực vật phổ biến nhất được hươu la tiêu thụ là:
Hươu la cũng ăn cỏ lạc mang, cỏ cách lan mã, cỏ tước mạch, và cỏ vũ mao, cũng như hoa bụi linh dương, hùng quả, anh đào đắng, hoa bụi đắng, sồi đen, dẻ ngựa California, ceanothus, tuyết tùng, hồng bụi, hoàng cận, thù du sông, hoàng liên gai leo, sơn thù du, thông Douglas, quả cơm cháy, fendlera, hoa mắt vàng, mận chuột lá nhựa, thông lá ngắn, chút chít, kohleria, manzanita, mesquite, sồi, thông, cúc bụi thỏ, cỏ phấn hương, quả mọng đỏ, sồi bụi, hoa đường lệ (gồm có đường lệ Thái Bình dương), bách xù Sierra, tua lụa, mao hạch, cỏ cảnh thiên, hướng dương, tesota, cỏ ngấy hương, sồi xám, cơm cháy nhung, anh đào dại phương tây, anh đào đen, và yến mạch hoang dã.[19] Nơi có sẵn, hươu la cũng ăn nhiều loại nấm hoang dã, phong phú nhất vào cuối mùa hè và mùa thu ở vùng núi phía nam Rocky. Nấm cung cấp hơi ẩm, protein, phốt pho và kali.[14][19]
Con người đôi khi tham gia vào nỗ lực bổ sung thức ăn trong mùa đông khắc nghiệt trong một có gắng tránh nạn đói hươu la. Cơ quan động vật hoang dã không khuyến khích những nỗ lực đó nhất, có thể gây hại cho quần thể hươu la do lây lan bệnh (ví dụ như lao và bệnh thoái hóa kinh niên) khi hươu tụ tập ăn; phá vỡ mô hình di cư; gây quá tải số lượng của quần thể hươu la địa phương và vượt quá chồi non cây bụi và thảo mộc.[20] Nỗ lực bổ sung thức ăn phù hợp khi tiến hành cẩn thận trường hợp hạn chế, nhưng để thành công khi cho ăn phải bắt đầu sớm trong mùa đông khắc nghiệt, trước khi điều kiện phạm vi kém và thời tiết gây ra suy dinh dưỡng hoặc nạn đói nghiêm trọng, phải được tiếp tục cho đến khi điều kiện phạm vi có thể hỗ trợ bầy đàn.[20]
Hươu la có thể được chia thành hai nhóm chính: Hươu la (sensu stricto) và hươu đuôi đen. Nhóm thứ nhất bao gồm tất cả các phân loài, ngoại trừ O. h. columbianus và O. h. sitkensis, đó là trong nhóm hươu đuôi đen.[3] Hai nhóm chính từng được xem như loài riêng biệt, nhưng chúng lai giống, và hầu như tất cả nhà chức trách gần đây xem xét hươu la và hươu đuôi đen như cùng loài.[1][3][4][5][7][21] Dường như hươu la tiến hóa từ hươu đuôi đen.[7] Mặc dù vậy, mtDNA của hươu đuôi trắng và hươu la tương tự nhau, nhưng có sự khác biệt so với hươu đuôi đen.[7] Điều này có thể là kết quả của sự pha trộn gen, mặc dù giống lai giữa hươu la và hươu đuôi trắng hiếm trong tự nhiên (dường như phổ biến hơn tại địa phương tây Texas), và tỷ lệ sống con lai thấp ngay cả trong điều kiện nuôi nhốt.[6][7] Nhiều tuyên bố quan sát giống lai tự nhiên không hợp pháp, như nhận dạng dựa trên đặc điểm bên ngoài khá phức tạp.[6]
Một số nhà chức trách đã công nhận O. h. crooki là một danh pháp đồng nghĩa của O. h. eremicus, nhưng kiểu mẫu vật xưa cũ là một con lai giữa hươu la và hươu đuôi trắng, vì vậy danh pháp O. h. crooki không hợp lệ.[3][22] Ngoài ra, tính hợp lệ của O. h. inyoensis được đặt câu hỏi, hai danh pháp hươu phân bố biển đảo O. h. cerrosensis và O. h. sheldoni có lẽ là đồng nghĩa với O. h. eremicus hoặc O. h. peninsulae.[21]
10 phân loài hợp lệ dựa trên ấn bản thứ ba quyển sách Mammal Species of the World (Loài hữu nhũ thế giới) là:[3]
Hươu la (tiếng Anh: Mule deer, danh pháp hai phần: Odocoileus hemionus), là một loài hươu thuộc chi Odocoileus, họ Cervidae, phân họ Capreolinae, bộ Artiodactyla.
Đây là loài hươu bản địa tại phía tây Bắc Mỹ. Được đặt tên theo hình dạng lỗ tai, lớn giống như con la. Được cho là có vài phân loài, bao gồm cả hươu đuôi đen.
Không giống như họ hàng hươu đuôi trắng (Odocoileus virginianus), hươu la gắn liền với vùng đất bờ tây sông Missouri, đặc biệt tại dãy núi Rocky của Bắc Mỹ. Hươu la cũng được du nhập đến Argentina.
Имея большие уши и прекрасное зрение, самцы могут заметить появление другого животного на расстоянии до 600 м. При появлении опасности, они могут попытаться спрятаться или сделать угрожающую позу в надежде перехитрить хищника. Они также могут убежать в другое место ещё до того, как хищник их заметил, или по-возможности быстро забраться высоко в гору, чтобы хищник посчитал эту добычу слишком тяжёлой для себя. Наконец, при близкой опасности олень делает непредсказуемые броски в разные стороны или ищет преграду между собой и хищником. Олень чернохвостый хорошо плавает, но редко использует источники воды при бегстве.
Олень чернохвостый является жвачным животным, и его популяция напрямую зависит от наличия удобоваримых сочных кормов. Диета оленя состоит из приблизительно равной пропорции деревянистых веточек и трав. Они также питаются желудями, бобами и свежими фруктами, включая ягоды и косточковые плоды, которые богаты клетчаткой и хорошо перевариваются.
Брачный период у чернохвостых оленей происходит в конце ноября — середине декабря, в это время самцы выясняют между собой отношения в борьбе за самку. Беременность длится 204 дня, пик появления детёнышей наступает в середине июня — начале июля. Молодые оленята весят 2-5 кг, как правило детёныши-самцы крупнее.
Различают следующие подвиды оленя чернохвостого:
Имея большие уши и прекрасное зрение, самцы могут заметить появление другого животного на расстоянии до 600 м. При появлении опасности, они могут попытаться спрятаться или сделать угрожающую позу в надежде перехитрить хищника. Они также могут убежать в другое место ещё до того, как хищник их заметил, или по-возможности быстро забраться высоко в гору, чтобы хищник посчитал эту добычу слишком тяжёлой для себя. Наконец, при близкой опасности олень делает непредсказуемые броски в разные стороны или ищет преграду между собой и хищником. Олень чернохвостый хорошо плавает, но редко использует источники воды при бегстве.
Олень чернохвостый является жвачным животным, и его популяция напрямую зависит от наличия удобоваримых сочных кормов. Диета оленя состоит из приблизительно равной пропорции деревянистых веточек и трав. Они также питаются желудями, бобами и свежими фруктами, включая ягоды и косточковые плоды, которые богаты клетчаткой и хорошо перевариваются.
Брачный период у чернохвостых оленей происходит в конце ноября — середине декабря, в это время самцы выясняют между собой отношения в борьбе за самку. Беременность длится 204 дня, пик появления детёнышей наступает в середине июня — начале июля. Молодые оленята весят 2-5 кг, как правило детёныши-самцы крупнее.
约10种
騾鹿(學名:Odocoileus hemionus)因有像騾的耳朵而得名,又稱做黑尾鹿,分布在北美西部的草原、農地到林地邊緣。毛皮在夏天為鏽棕色,冬天轉為灰棕色。

 分類 界 : 動物界 Animalia 門 : 脊索動物門 Chordata 亜門 : 脊椎動物亜門 Vertebrata 綱 : 哺乳綱 Mammalia 目 : ウシ目 Artiodactyla 亜目 : 反芻亜目 Ruminantia 科 : シカ科 Cervidae 亜科 : オジロジカ亜科 Odocoilinae 属 : オジロジカ属 Odocoileus 種 : ミュールジカ O. hemionus 学名 Odocoileus hemionus 和名 ミュールジカ 英名 Mule deer
分類 界 : 動物界 Animalia 門 : 脊索動物門 Chordata 亜門 : 脊椎動物亜門 Vertebrata 綱 : 哺乳綱 Mammalia 目 : ウシ目 Artiodactyla 亜目 : 反芻亜目 Ruminantia 科 : シカ科 Cervidae 亜科 : オジロジカ亜科 Odocoilinae 属 : オジロジカ属 Odocoileus 種 : ミュールジカ O. hemionus 学名 Odocoileus hemionus 和名 ミュールジカ 英名 Mule deer ミュールジカ(Odocoileus hemionus)は、北アメリカ西部で普通にみられるシカである。耳がラバ(ミュール)のそれに似ているのでこの名前がある。尻尾がすべて黒いオグロジカを含む数種類の亜種があり、近縁のオジロジカとは違って、ミズーリ川より西の地域、特に、ロッキー山脈付近に分布する。ハワイのカウアイ島やアルゼンチンに移入されたこともある[1]。
シートン動物記の The Trail of The Sandhill Stag(邦題『サンドヒルの牡鹿』『サンドヒル・スタッグ どこまでも続く雄ジカの足あと』)に出てくるのは、このミュールジカである[2]。
オジロジカ属の中では最大種で、肩高1-1.1m、体長2mにもなる。大人のオスは体重が68kgから140kg、一方メスは平均して57kgから79kgで、大きなオスになると230kgにもなる[3]。オジロジカのように、同じ地域内で際立った体格差が見られることはない。
オジロジカとの外見上の大きな違いは、耳の大きさ、尻尾の色、そしてオスの角の形である。体の大きさの違いで見分けられることも多い。また、ミュールジカの尻尾は先端が黒いが、オジロジカは黒くない。角も、ミュールジカは複数の枝に分かれ、年を重ねるごとにフォーク状に横に張るが、オジロジカの角は、1本の角から枝分かれする。
角は毎年、交尾期の後に抜け落ちるが、春にはすぐに新しいものが生えて来る。抜け落ちるのは2月半ばが一般的だが、地域差がある[4]。
夏は主に草を食べるが、果実を食べることもある。例えばブラックベリー、ブルーベリー、レモンリーフの実やスィンブルベリーである。また、有毒であるにもかかわらず、カリフォルニア・バックアイ(カリフォルニアトチノキ)の葉を食べることでも知られる。
冬は毬果、とりわけダグラスモミや、ヒノキ科や、イチイ属、ビャクシン属の樹木の実を食べる。また、落葉樹や灌木(特にハコヤナギ、ヤナギ、ハナミズキ、ザイフリボク、セージ)の枝も食べる。ドングリやリンゴを食べることもある。ミュールジカが生息する地域の大部分は、冬になると、食物が雪と氷におおわれ、そうでないわずかな食物も成長が鈍る。食物が少ない環境での冬を乗り切るために、ミュールジカは動きが少なくなり、新陳代謝が鈍くなる。冬はミュールジカの死亡率が一番高くなる時期で、特にその前の春に生まれた子の死亡率が高い。そのため、ミュールジカは、冬場は雪が多い山から谷へと移動する。人間による餌付けが行われることもあるが、こういう給餌のやり方は、よほど適切に行われない限り、いい結果を生まない。
[5][6]
ミュールジカは木の葉よりも草を食べる傾向があり、食物となる草の多寡によって生息数が増減する。水や餌を求めて移動することはまれで、食物があるところから、歩いて移動できる場所にねぐらを構える性質がある。
子供のころは家族単位で採食するが、大人のオスは単独、もしくは他のオス数頭と採食する。夜明けまたは日暮れの頃に食物を採り、昼間は休んでいることが多いが、見通しのいい農地や、満月の時期、そして、ハンターから追われている場合には、夜にも餌をあさる。ミュールジカの寝る場所は、たらいほどの大きさで、よく使う場所は表面がこすり取られているが、間に合わせのねぐらは、せいぜい草がぺしゃんこになった程度である[7]。
繁殖のサイクルも、このシカを知る上では欠かせない。交尾期もしくは発情期は秋に始まり、メスは数日間盛りがついた状態となり、オスは、相手を求めてより攻撃的になる。メスは最低1頭のオスと交尾するが、つがいとならなかった場合は、一月以内に発情期に戻る。妊娠期間は約190-200日で、春に生まれた子ジカは、生後60-75日経って乳離れをする。
子ジカは通例1頭または2頭だが、たまに三つ子が誕生することもある。子ジカは、生まれた年の冬は母親と一緒に過ごす[8]。
ミュールジカの天敵は人間に留まらない。ハイイロオオカミやクーガーは、成獣にとっての主な天敵である。ボブキャット、コヨーテ、アメリカクロクマやハイイログマは、成獣はめったに捕食しないが、子ジカは普通に捕食する[5]。
1960年代より、プリオンによる慢性消耗病(CWD)が問題になっている[9]。
ミュールジカは、狭義のミュールジカ(sensu stricto)とオグロジカの2つのグループに分けられる[1]。オグロジカの仲間である O. h. columbianus と O. h. sitkensis を除くすべての亜種が、ミュールジカの仲間に含まれる。この両者は別亜種として扱われているが、交配が可能であり、最近では、専門家はこの2種をほぼ同種として扱っている[10] 。
ただし、ミュールジカとオジロジカのミトコンドリアDNAは似通っている一方で、ミュールジカとオグロジカのミトコンドリアDNAには比較的大きな違いがある。これは交雑による遺伝子汚染のせいだと考えられているが、ミュールジカとオジロジカが野生の状態で交雑することはまれで、交雑種は生存率が低い(ただしテキサス西部では、一般的になっているようである)。交雑の観察記録の主張は正当とは言えず、外見的な特徴による識別は難しい[10]。
ミュールジカ(Odocoileus hemionus)は、北アメリカ西部で普通にみられるシカである。耳がラバ(ミュール)のそれに似ているのでこの名前がある。尻尾がすべて黒いオグロジカを含む数種類の亜種があり、近縁のオジロジカとは違って、ミズーリ川より西の地域、特に、ロッキー山脈付近に分布する。ハワイのカウアイ島やアルゼンチンに移入されたこともある。
シートン動物記の The Trail of The Sandhill Stag(邦題『サンドヒルの牡鹿』『サンドヒル・スタッグ どこまでも続く雄ジカの足あと』)に出てくるのは、このミュールジカである。