fr
noms dans le fil d’Ariane


Anolis carolinensis is equipped with certain communication signals from birth. Most communication involves color variations, actions such as head bobbing or neck biting, or use of the dewlap. The dewlap is used for inter-gender communication, especially during the breeding season. Displaying the dewlap also may be used to determine competitive status between males; in these cases, dewlap displays are usually related to territorial boundary disputes. Head bobbing or courtship bobbing is performed by both males and females to communicate breeding status, but is also done while in a threatened state.
Green anoles that have not yet reached adulthood do display adult signals and behaviors (e.g. head bobbing). However, since they are not sexually mature, these do not function as courtship mechanisms. Interactions between juveniles are similar to those of adult females. They generally are not as serious as those between adult males and usually do not result in injuries. As juveniles mature, their interactions often become more intense. This is mainly due to the development of structural hierarchies for adulthood.
Communication Channels: visual ; tactile
Perception Channels: visual ; tactile
Anolis carolinensis is currently considered to be at lower risk or of least concern and is not vulnerable to any major threats at this time. Some researchers believe that they may be at risk due to the significant numbers in the pet trade. However, in recent years, sales of green anoles have declined due to lesser demand. Also, green anoles appear abundant in the portions of their range from which they are collected and many populations occur in protected areas, such as parks and natural areas, which helps to protect the population.
US Federal List: no special status
CITES: no special status
IUCN Red List of Threatened Species: least concern
After a female lays her eggs, a five to seven week gestation period is necessary. Green anoles have genotypic sex determination. Once the young hatch from their eggs they resemble adults in coloration and pattern, but are only 23 to 25 mm long. Green anoles have determinate growth; they grow at a relatively constant rate from hatching to adulthood. Hatchlings develop into juvenile males and females without any parental investment. Juvenile males and females have the same resource and survival needs while developing, but competition among juveniles is generally low because resources tend to be plentiful. Since juveniles are not sexually mature, their main activities are associated with foraging, protection against predators, and maintaining adequate body temperatures. Initially, juvenile male and females do not show any sexual differences or display behaviors; however, during later stages of development, testosterone levels become higher in males and are likely to exhibit more aggressive behaviors.
Unlike other Anolis species, such as Anolis aeneus, green anoles do not leave their hatch sites after breeding.
There are no known adverse effects of Anolis carolinensis on humans. Anolis carolinensis is a relatively harmless reptile. It is not aggressive toward humans, and its bite force is most likely insufficient to damage human skin.
One of the best known positive economic factors involving green anoles are their presence in the pet trade. Green anole are sold in many pet stores in the United States. They also are exported for profit. In addition, lizards collected within the United States are sold to zoos and for educational programs. Green anoles also have been studied to better understand animal behavior.
Green anoles also are sometimes considered beneficial pest controllers, because they feed on pest species such as spiders, moths, and crickets.
Positive Impacts: pet trade ; research and education; controls pest population
Anolis carolinensis does not have a large ecosystem impact in most geographical ranges. However, their introduction in the Ogasawara Islands of Japan, however, has led to the decline of or extinction of many species, such as the Ogasawara tumbling flower beetle (Glipa ogasawarensis). In other regions, its greatest impact is as a prey species. For example, in Guam, green anoles are so heavily preyed upon by brown tree snakes (Boiga irregularis) that they have been nearly extirpated from the area.
Because A. carolinensis is highly territorial, especially the males, they may prevent certain other species from entering their territory. This potentially prevents certain reproductive variation. A beneficial quality of green anoles is that they consume seeds and grains, potentially aiding in seed dispersal.
Ecosystem Impact: disperses seeds
Green anoles feed on a broad range of prey items. They often will attempt to eat anything smaller than their own head. They are classified as insectivores, eating a wide variety of insects, including beetles and flies, as well as spiders, some arthropods. At times, they also will eat mollusks, grain, and seeds. The importance of a particular prey or food item largely reflects its availability. If an item is abundant within the territory, green anoles are likely to feed on it more frequently.
Green anoles have several methods of capturing prey. Over 58% of the prey is captured by perching and watching or anticipating prey until they are within striking distance. This is considered to be the most effective means of capturing prey. This behavior is predominant during breeding, to conserve energy for mating. Another method of prey capture is used while the anole is protecting and patrolling their territory. In this case, they leap forward to ensure a capture, but use a slower motion. Another common method of prey capture is the ambush, usually used in capturing larger prey items.
Animal Foods: insects; terrestrial non-insect arthropods; mollusks
Plant Foods: seeds, grains, and nuts
Primary Diet: carnivore (Insectivore )
Anolis carolinensis (green anoles) is native to neotropical and nearctic regions. Anolis carolinensis occurs throughout much of the southeastern United States, extending north through parts of North Carolina, west to Texas, and south through Florida. While Florida was once the central portion of its United States distribution, today most Florida populations have been replaced by introduced anole species, such as Anolis sagrei.
In other parts of its geographic range, A. carolinensis is considered an introduced species. It has become abundant in Hawaii since it was discovered in 1950. It also has been introduced and has flourished in the Ogasawara Islands of Japan, and in Cuba, the Bahamas, and Guam. In Guam, however, densities have been impacted drastically by predators, such as introduced brown tree snakes (Boiga irregularis).
Biogeographic Regions: nearctic (Native ); palearctic (Introduced ); neotropical (Introduced , Native ); oceanic islands (Introduced )
Anolis carolinensis is a primarily arboreal lizard. Within natural habitats, A. carolinensis is found most often on shaded tree branches. Its positioning within a tree is known as its perch height and is dependent on the proximity of both predators and prey. Limited research has been done on their preferred types or species of trees. Anolis carolinensis appears mostly to inhabit trees and shrubs within their territory and where prey is readily available. They also are frequently observed in tall grasses.
Anolis carolinensis also is one of the most common lizards in urban and suburban areas. It is frequently found near dwellings, particularly on fence posts and the sides of buildings.
Range elevation: 45.73 to 609.76 m.
Average elevation: 327.75 m.
Habitat Regions: temperate ; tropical ; terrestrial
Terrestrial Biomes: savanna or grassland ; forest
Aquatic Biomes: coastal
Other Habitat Features: urban ; suburban
Green anoles have a lifespan ranging from 2 to 8 years, determined largely by predation. Lifespan in captivity is similar to that in the wild, approximately 4 to 6 years, and dependent on proper care and conditions. Longevity also is greatly dependent upon proper nutrition. Smaller, slower, green anoles potentially have greater difficulty obtaining necessary nutrients than larger individuals, especially if engaged in competition. Larger green anoles under ideal natural conditions have been known to live up to 10 years.
Range lifespan
Status: wild: <1 to 10 years.
Average lifespan
Status: wild: 5.5 years.
Range lifespan
Status: captivity: 1.5 to 7 years.
Typical lifespan
Status: wild: 2 to 8 years.
Typical lifespan
Status: captivity: 2 to 7 years.
Anolis carolinensis varies in length from 4 to 8 cm. Females typically are smaller in all body size measures, at birth ranging from about 23 to 25 mm long. Both males and females have long tails that account for more than half of their total body lengths. Adult anoles weigh between 2 and 6 g.
Scale colors in green anoles vary. In most cases, these lizards range from shades of brown to green or gray. At times their coloring represents combinations of these colors. Color variation results from layers of pigmented cells called chromatophores. Three types of pigment cells are present: xanthophores, cyanophores, and melanophores, each responsible for different color variations. Green anoles are capable of changing scale color in response to their external environment. Many factors affect color change and variation; most often it is dependent upon temperature and excitation, such as increased activity or competition. Darker brown and black colors, produced by melanophores, typically signal cold or stressed conditions.
Within a population, two different size classes or morphs of adult males may be present: heavyweights and lightweights. These morphs differ in many ways, including bite force, body mass and length, competition, and vertical jump. The heavyweight morph is larger and more dominant. Some authors consider these morphs to be different developmental stages or different age classes among sexually mature males.
Physical differences also are common between males and females. Females often have a line that runs along their dorsal surface, from their neck down to their back, ending before their tail begins. Most males have dewlaps that extend from the ventral side (underneath) of their neck. Dewlaps are rarely seen in females. The dewlap is commonly pinkish in color and thought to be used by males to increase visibility as they court females. Displaying the dewlap may also represent a competitive status between males; in these cases, dewlap displays are usually related to territory boundary disputes. Subspecies Anolis carolinensis seminolus, abundant in southwest Florida, is physically very similar to A. carolinensis carolinensis, but its dewlap is often white or gray.
Range mass: 2 to 6 g.
Range length: 10.16 to 20.32 cm.
Other Physical Features: heterothermic
Sexual Dimorphism: male larger; sexes colored or patterned differently; ornamentation
Green anoles are preyed upon by a relatively large assortment of predators. Their main predators are snakes and birds, but they also are preyed on by larger reptiles. Brown tree snakes (Boiga irregularis) are particularly common snake predators. This species has eliminated green anoles from portions of Guam. Examples of birds that regularly prey on green anoles are American kestrels (Falco sparverius), pearly-eyed thrashers (Margarops fuscaturs), and lizard cuckoos (Saurothera vieilloti). A larger reptile that preys on green anoles is the curly tailed lizard (Leiocephalus carinatus). Other common predators, particularly in suburban areas, are cats, dogs, and frogs.
To avoid predators, green anoles hide in trees, tall grasses, and other vegetation. They also have developed a structure similar to a patagium that enables them to glide down from tall trees. In addition, green anoles have the ability to walk vertically on surfaces such as trees, walls, and fences using adhesive pads on the bottom of their feet. These provide a means of escape that the majority of their predators do not have.
Green anoles also utilize caudal autotomy and use their dropped tails to distract predators while they escape.
Known Predators:
The majority of green anoles are polygynous. Especially in larger populations, they usually will mate only within their own territories. Females are not characteristically known to search for different mates. In cases when a female mates with a different male, it is usually due to intrusion into her territory.
Green anoles breed roughly four to five months out of the year, usually from April through August. Warmer months have the highest reproduction rate, because higher temperatures increase the size of male and female sexual structures (testes and ovaries). Ovulation cycle for female green anoles lasts approximately two weeks, which creates the intervals in which they mate.
The sexual display behavior of green anoles is very specific. Members of almost every mating pair live within each others territory. To attract the attention of females, males bob their heads up and down and extend their dewlaps. Not all females are receptive to male courtship; some deny them and others exhibit the same behavior as males but then arch their neck to inform the males they are receptive to mating. The male then approaches the female and bites the back of her neck, a distinctive behavior of green anoles. The male stabilizes himself by positioning his tail beneath the female’s body and then mounts her back. The male’s himepenes are at the base of his tail. Once in position, he will insert these into the female’s cloaca. Mating typically lasts only a few minutes.
Males protect their mating partners from other intruding males by defending their territory. At times, males have been found to deny receptive females due to their focus on territorial protection. Females also show protective behavior by mating primarily in sheltered areas and closed terrain, reducing vulnerability to predators. Unlike other Anolis species, such as Anolis aeneus, green anoles do not leave their hatch sites after breeding.
Mating System: polygynous
Breeding period for A. carolinensis occurs during warmer months, generally April through August. The breeding intervals are based on the female reproductive cycle, as they are only receptive to mating during their ovulatory cycle. The male is the main initiator of reproductive interactions and presents a strong display of attraction. This typically promotes a reproductive state in the female, similar to that of Anolis aeneus. Depending on how many ovulatory cycles a female has within a breeding season, she will lay six to nine eggs in a year. On average, she will lay a one to two egg clutch every two weeks. The male’s opportunities for mating correlates with the number of ovulation cycles a female has and the total number of potential mates within his territory range.
Two types of sexual selection occur during the mating season: intersexual and intrasexual selection. The larger a territory range a male has, the more females he is likely to mate with. A territory size usually relates to a male green anoles body size; the larger he is the more dominant he will be towards intruders and predators as he protects his territory.
Female green anoles have the ability to store sperm; this may be a trait of intersexual selection. Sperm has been found within a female seven months after mating, which may make delayed fertilization possible. Prior to releasing her clutch, the female will examine an appropriate area and then dig into the soil. Females prefer to release their eggs into moist soil. Eggs are oval and on average 6 by 4.5 mm. The gestation period varies, but is approximately five to seven weeks long. Hatchling anoles weigh 0.27 g each. Juvenile anoles are sexually mature at 8 to 9 months old.
Breeding interval: Green anoles breed in two week intervals throughout the spring to summer months.
Breeding season: Green anoles breed 4 to 5 months out of the year, usually April through August.
Range number of offspring: 6 to 9.
Range gestation period: 5 to 7 weeks.
Range age at sexual or reproductive maturity (female): 8 to 9 months.
Range age at sexual or reproductive maturity (male): 8 to 9 months.
Key Reproductive Features: iteroparous ; seasonal breeding ; sexual ; oviparous ; sperm-storing ; delayed fertilization
After ovulation, fertilization, and egg laying, no parental investment is known to occur.
Parental Investment: no parental involvement; pre-fertilization (Provisioning, Protecting: Female)
Anolis carolinensis or green anole (US: /əˈnoʊ.li/ (![]() listen)) (among other names below) is a tree-dwelling species of anole lizard native to the southeastern United States and introduced to islands in the Pacific and Caribbean. A small to medium-sized lizard, the green anole is a trunk-crown ecomorph and can change its color to several shades from brown to green.
listen)) (among other names below) is a tree-dwelling species of anole lizard native to the southeastern United States and introduced to islands in the Pacific and Caribbean. A small to medium-sized lizard, the green anole is a trunk-crown ecomorph and can change its color to several shades from brown to green.
Other names include the Carolina anole, Carolina green anole, American anole, American green anole, North American green anole and red-throated anole. It is commonly called chameleon in the southeastern United States and sometimes referred to as the American chameleon (typically in the pet trade) due to its color-changing ability; however, it is not a true chameleon.
The green anole is a small to medium-sized lizard, with a slender body. The head is long and pointed with ridges between the eyes and nostrils, and smaller ones on the top of the head. The toes have adhesive pads to facilitate climbing. They exhibit sexual dimorphism, the males being fifteen percent larger.[3] Adult males within a population can be classified within a heavyweight and a lightweight morph.[4] The male dewlap (throat fan) is three times the size of the female's and bright orange to pink, whereas that of the female is lighter in color. The dewlap is usually pink for Anolis carolinensis (more orange-red in A. sagrei) and is very rarely present in females. The color of the dewlap is variable and different from the lizard eye to the human eye. Green anoles are thought to be capable of seeing a larger range of the UV spectrum, and that the dewlap reflects ultraviolet light for attracting mates.[5] Female anoles do, however, often have a dorsal line down their back. Extension of the dewlap from the throat is used for communication. Males can form a pronounced dorsal ridge behind the head when displaying or when under stress. Females and juveniles have a prominent white stripe running along their spine, a feature most males lack.[6]
Adult males are usually 12.5–20.3 cm (4.9–8.0 in) long, with about 60-70% of which is made up of its tail, with a body length up to 7.5 cm (3.0 in) and can weigh from 3–7 g (0.11–0.25 oz).[3][7][8][9]
Colour varies from brown to green and can be changed like many other kinds of lizards, but anoles are closely related to iguanas[10] and are not true chameleons. Although A. carolinensis is sometimes called an 'American chameleon', true chameleons do not naturally occur in the Americas, and A. carolinensis is not the only lizard currently in its area of distribution capable of changing colour. In contrast, many species of true chameleons display a greater range of color adaptation, though some can hardly change color at all.[3][11]
Typical coloration for a green anole ranges from bright green to dark brown, with little variation in between. The color spectrum is a result of three layers of pigment cells or chromatophores: the xanthophores, responsible for the yellow pigmentation; cyanophores, responsible for the blue pigmentation, and melanophores, responsible for the brown and black pigmentation. The anole changes its color depending on mood, level of stress, activity level and as a social signal (for example, displaying dominance). Anolis carolinensis takes darker coloration as its base color at the beginning of the breeding season when it is generally cooler, and the adult males change their body coloration to more greenish when they need to advertise their territorial possession.[12] Although often claimed, evidence does not support that they do it in response to the color of the background (camouflage).[13][14] Whether they do it in response to temperature (thermoregulation) is less clear, with studies both supporting it[15] and contradicting it.[16] Changing color while under a sharply contrasting shadow can cause a "stencil effect", where the outline of the shadow is temporarily imprinted in the animal's coloration (see image in gallery, below). When stressed—while fighting, for example—the skin just behind the lizard's eyes may turn black independently from the rest of the animal's coloration, forming "postocular spots".
A lack in one of the pigment genes causes color exceptions. These color mutations are also called phases. The rare blue-phased green anole lacks xanthophores, which results in a blue, rather than red, often pastel blue, anole. These specimens have become popular recently in the pet trade market. When the anole is completely lacking xanthophores, it is said to be axanthic and the animal will have a completely pastel- or baby-blue hue. They are extremely rare—usually produced in one of every 20,000 individual anoles in the wild. Another phase is the yellow-phased green anole, which lacks cyanophores. Colonies of these rare color-phased anoles have been reported, but anoles with these color mutations rarely live for long, since the green color provides camouflage for hunting down prey, as well as hiding from predators.
Anolis carolinensis is a species of the large lizard genus Anolis within the family Dactyloidae (anole lizards). Within the genus, thirteen species have been identified as a distinct clade, referred to as the Anolis carolinensis series. This group are mid-sized trunk crown anoles with large conspicuously elongated heads and extreme levels of sexual dimorphism. The species was named by Friedrich Siegmund Voigt (1781 - 1850) in 1832.[2]
This species is native to North America, where it is found mainly in the subtropical southeastern parts of the continent. Anoles are the most abundant on the Atlantic Coastal Plains in North Carolina, South Carolina, Georgia and Florida, and on the Gulf Coast in Alabama, Mississippi, Louisiana, and Texas, where they extend inland as far as Texas Hill Country and the DFW Metroplex; they have also been recorded in Tamaulipas, Mexico, but it is mostly likely an introduction. In the Carolinas, they are found on the coastal plains as far north as False Cape in Virginia,[17] and in the southern piedmont of North Carolina, but throughout South Carolina,[11] while in Georgia they are widespread except in the Blue Ridge region.[3]
The species has been introduced into various locales in the Pacific and the Caribbean: Hawaii, the Ogasawara Islands, the Northern Mariana Islands, the Bahamas, Anguilla, Palau, and Saint Vincent and the Grenadines, as well as the Canary Islands. In 2005 they were recognized and listed as an invasive alien species in the Ogasawara Islands of Japan for causing insect population collapse.[18] They have been sighted in Orange County and San Diego County of southern California, with sightings in San Diego going at least as far back as 1993.[19]
A. carolinensis is arboreal in nature but may be seen on the ground and frequently seen on shrubs in the low country of the Carolinas. However, it can live in cities like Atlanta with little trouble so long as there is plentiful vegetation and bugs to eat. One can observe them on steps, trellises, and railings adjacent to foliage; on particularly hot summer days they may seek to cool off on indoor walls or on wrap around porches of older buildings, and in the former case can simply be captured in a shoebox and gently placed outdoors. It is common on roadsides, the edges of forests where there are shrubs and vines, but also construction sites having abundant foliage and sunlight. Their preferred habitat is open pine communities with a greater shrub density, it may harbor a greater abundance of anoles [20] where they are able to watch for prey and intruders coming into their territory.
Although not threatened as a species, Carolina anoles increasingly struggle with competition from introduced anole species, such as the brown anole (Anolis sagrei), also known as the Bahamian anole. This competition happened to be an interesting model for evolutionary studies, as it illustrates the process of adaptation. When A. sagrei first appeared in the United States in the early 1900s,[21] the Carolina anole mostly ceded ground-level territories and were relegated to a very different ecosystem high in the treetops. On occasion, more aggressive Carolina anole individuals may still be seen closer to the ground. Currently, A. carolinensis is abundant in its area of distribution and is able to thrive in disturbed areas, so it is not considered threatened, but A. sagrei may represent a developing threat in some areas.[3]
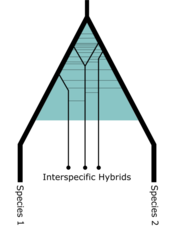
A. carolinensis has been found to regularly hybridize with a closely related species, Anolis porcatus (the Cuban green anole), in Southern Florida, where A. porcatus has been introduced.[22] A 2022 study found there to be asymmetric introgression of certain A. porcatus alleles within the population of hybrid individuals, three of which were found to be significantly associated with environmental variables indicative of urbanization.[22] It remains uncertain as to how this admixture of invasive alleles to the Carolina anole will affect the conservation of the species going forward.[22] Not all admixture from invasive populations should be viewed as a negative outcome, and adaptive introgression as a result of hybridization with an ecologically robust invasive population might facilitate the long-term survival of native populations otherwise unable to adapt to human impact on the environment.[22]
Male anoles are strongly territorial creatures. Some have even been witnessed fighting their own reflections in mirrored glass. The male will fight other males to defend his territory.[23] On sighting another male, the anole will compress his body, extend the dewlap, inflate a dorsal ridge, bob his head and attempt to chase the rival away. If the rival male continues to approach, anoles will fight by biting and scratching each other. Studies have also shown that there is a positive correlation between bite-force and the size of the individual's dewlap.[24]
One study showed that heavyweights had 50% higher testosterone concentrations than lightweights during the breeding season. It seems that disproportionally larger heads and dewlaps may be correlated to higher bite forces of heavyweights.[25] Those with darker colorations will choose lower perch sites compared to their lighter conspecifics.[26] For heavyweight males of the same size the one with the higher bite force wins disputes more frequently.[27]
Adult female anoles have much smaller dewlaps that they rarely use during encounters with other anoles and never use during courting.[28]
Hormones, sexual signals, and performance of green anole lizards (Anolis carolinensis),
[29] Serious injury is rare, but males often carry numerous scars on their heads and faces, especially during the mating season. Their territories, which are about 1 m3 (35 cu ft), usually include two to three females.[3][11]
The Carolina anole is diurnal and active throughout the year, peaking in spring and fall. Winter activity is dependent on sun and temperature.[3]
An anole's diet consists primarily of small insects such as crickets, grasshoppers, flies, butterflies, moths, cockroaches, small beetles, and other arthropods, including spiders, as well as occasionally feeding on various molluscs,[30] grains, and seeds.[3][11] Although anoles have been observed preying upon smaller reptiles such as juvenile skinks, this is not thought to be typical behavior.[3] Many people who keep these lizards as pets feed them mealworms, grubs, maggots, and small crickets.
Major predators include the broadhead skink, snakes, birds, and in urban habitats, cats. Like many lizards, anoles display autotomic tails, which wiggle when broken off. This distracts the predator and helps the anole to escape. A new tail then starts to develop.[3] The new tail, however, containing cartilage rather than bone, will typically not grow back to the same length as the first one, and may exhibit a marked difference in color and texture from the rest of the animal. Green anoles will also try to escape predators by climbing vertical walls, trees, fences, or any vertical surface they can find. This ability is possible due to their enlarged toe pads and great climbing ability.[31]
Anoles are parasitized by some species of sarcophagid flies, including Lepidodexia blakeae.[32] Adult flies will deposit eggs on live anoles, and the fly larvae develop inside the lizard until they emerge from a wound and pupate into adult flies in sediment. Infection is often fatal, with mortality rates possibly as high as 90%.[32]
The typical breeding season for Carolina anoles starts as early as April and ends in late September, gonadal activity being largely regulated by photoperiod, enlarging in spring as the weather warms up and days lengthen, and then regressing in late summer.[3][11]
During this time, the males patrol their territory and the most brilliant displays of these creatures can be seen. Males defend their territory and females from rivals, while courting the females with elaborate displays of extending their brightly colored dewlaps while bobbing up and down, almost doing a dance.[33] The dewlap is also used to ward off other males. The male courts and pursues a female until the two successfully mate. Usually, when the female is ready to mate, she may let the male catch her, at which point he will grasp her by biting a fold of her skin behind her neck. The male will then position his tail underneath the female's tail near her vent. Males have two sex organs, known as hemipenes, which are normally kept within the body, but are everted from his vent for mating. Males seem to alternate between the left and right hemipenis on successive matings.[34]
The female matures one ovarian follicle at a time, the ovaries alternating in production. The sight of a courting male induces ovarian development, sexual receptiveness and then ovulation. About two to four weeks following mating, the female lays her first clutch of eggs, usually one or two in the first clutch. She can produce an egg every two weeks during the breeding season, until about 10 eggs have been produced. However, she can store sperm for up to eight months following mating. She then buries the soft-shelled eggs in a shallow depression in soft soil, leaf litter, compost, rotting wood, or even a hole in a nearby tree. Eggs average 12.5 mm (0.49 in) by 9.3 mm (0.37 in) in size.[3]
The eggs are left to incubate by the heat of the sun, and if successful, will hatch in about five to seven weeks (30–45 days) from late May to early October. The incubate temperature has to be 80 to 85 degrees Fahrenheit. On hatching, the hatchlings are 52–67 mm (2.0–2.6 in) in length.[3][11]
The hatchlings must fend for themselves, as they are not cared for by either parent. The young hatchlings must be wary of other adult anoles in the area, as well as larger reptiles and mammals, which could eat them. Younger anoles differ from adults in having less obvious head ridges, a wider head and shorter tail. They mature in about eight months.[3]
Carolina anoles' nervous natures makes it advisable not to attempt to handle them very often; despite this, Carolina anoles are popular pets. Individual animals may or may not adapt readily to cage life. Care must be taken to ensure the animals receive the support they need to adapt to captivity and live full and enriching lives; an adequately sized enclosure, as well as the appropriate plants and substrate material, are beneficial to the health of captive Carolina anoles.
A well-cared for green anole can be expected to live for up to 10 years, with longer being possible.[6]
This species has been chosen as a model reptile for genomics by the National Human Genome Research Institute genome sequencing program.[35] It was selected because of the ease and low cost of laboratory breeding and evolutionary value of the diversity of the genus.[36] In 2011, the complete genome of this lizard was sequenced and published in Nature.[37] Before its genome was published, only mammals and three bird species had been sequenced among amniotes.[38] The draft genome sequence is 1.78 Gb (compared with 2.0–3.6 Gb mammalian and 0.9–1.3 Gb avian genome assemblies), of which 27% are mobile elements such as LINEs. A total of 17,472 protein-coding genes and 2,924 RNA genes were predicted from the A. carolinensis genome assembly.[39]
O'Bryant, E. L., & Wade, J. (2001). Development of a sexually dimorphic neuromuscular system involved in green anole courtship behavior. Brain, Behavior and Evolution, 58(6), 362–369. https://doi.org/10.1159/000057577
Anolis carolinensis or green anole (US: /əˈnoʊ.li/ (![]() listen)) (among other names below) is a tree-dwelling species of anole lizard native to the southeastern United States and introduced to islands in the Pacific and Caribbean. A small to medium-sized lizard, the green anole is a trunk-crown ecomorph and can change its color to several shades from brown to green.
listen)) (among other names below) is a tree-dwelling species of anole lizard native to the southeastern United States and introduced to islands in the Pacific and Caribbean. A small to medium-sized lizard, the green anole is a trunk-crown ecomorph and can change its color to several shades from brown to green.
Other names include the Carolina anole, Carolina green anole, American anole, American green anole, North American green anole and red-throated anole. It is commonly called chameleon in the southeastern United States and sometimes referred to as the American chameleon (typically in the pet trade) due to its color-changing ability; however, it is not a true chameleon.