en
names in breadcrumbs


Oriental bittersweet uses both dioecious and perfect breeding systems [62]. This species is typically "functionally dioecious" because early abortion of either male or female organs makes most individual plants unisexual [10]. Plants occasionally develop both unisexual and perfect flowers, becoming polygamodioecious [42,129], and some plants are reportedly monoecious [58].
Hymenoptera, especially bees, pollinate Oriental bittersweet flowers. Wind pollination also occurs (fact sheet by [24], review by [170]).
Four Oriental bittersweet populations from Connecticut were studied for pollen viability. Mean pollen viability across populations was 67%, but viability was significantly different among populations (P<0.05), varying from 17.3% to 74.3%. Oriental bittersweet ? American bittersweet hybrids had low pollen viability [26].
This description covers characteristics that may be relevant to fire ecology and is not meant for identification. These sources: [42,64,110,114,129,173] provide general identification keys. Oriental bittersweet is commonly mistaken for the rarer, native American bittersweet [26]. See these sources: [41,89,106] for keys specifically designed to distinguish Oriental bittersweet from American bittersweet.
Morphology: Oriental bittersweet is a deciduous liana [175]. The stems are woody and twining [42,88,114,129]. They may reach 66 feet (20 m) in length and 4 inches (10 cm) in width [24,25,143], depending upon stem age and supporting vegetation [24]. In surveys along the plain of Lake Michigan (including sites in Illinois, Indiana, and Michigan), Oriental bittersweet stems were likely young, ranging from only 2.4 to 10.5 mm DBH [88]. The leaves are alternate, oblong, 2 to 5 inches (4-12 cm) long, and 1.4 to 2.0 inches (3.5-5.1 cm) wide [26,42,64,110,114]. Leaf morphology is highly variable [31,175], with Oriental bittersweet showing reduced leaf mass per unit leaf area and increased leaf area in shade [31]. Oriental bittersweet is functionally dioecious (see Breeding system). Flowers are sparse, occurring in 3-flowered, axillary cymes [26,42,64,110,114]. Fruits develop next to the vegetative buds [26]. Outer vegetative bud scales may be spiny [24]. A typical plant bears upwards of 370 fruits/year [173]. Fruits are dehiscent, 3-valved capsules about 0.4 inch (1 cm) in diameter [42,114,129,152]. The capsules are relatively large [30,120] and deciduous [173]. Each valve contains 1 or 2 seeds covered by fleshy, yellowish-red arils [42,114,129,152]. In Japan, Fukui [39] recorded mean sizes of 3.8 mm in seed length, 0.023 mm in seed width, and 7.5 mm in capsule length. Similar capsule sizes occur on plants in the United States (range: 1.5-1.6 mm in width; 6-8 mm in diameter) [13,24]. Oriental bittersweet roots are deep [139] and spreading [95]. They may be as much as 0.8 inch (2.0 cm) thick [95] and reach deeper than roots of surrounding plant species [139]. In greenhouse studies, biomass of Oriental bittersweet roots infected with mycorrhizae was less than biomass of uninfected roots (P≤0.05) [91].
Stand structure and age class: Oriental bittersweet's growth habit is climbing and/or sprawling. It uses woody shrubs and/or trees for structural support, intertwining its branches around support trunks and branches. Branches may eventually overtop or shade out supporting plants. In Rock Creek Park, Washington DC, Oriental bittersweet used other bole-climbing lianas and vines including Virginia creeper (Parthenocissus quinquefolia), poison-ivy, and English ivy (Hedera helix) for initial structural support. After twining around these lianas, Oriental bittersweet branches grew into and twined around tree crowns. This climbing habit enabled Oriental bittersweet to grow above other lianas and access the tops of the largest trees (3.7 feet (1.1 m DBH)) in the Park [128]. In red maple, American beech, red oak, and black oak forests on the plain of Lake Michigan, jack pine and white oak were more likely to support Oriental bittersweet than other overstory species (P<0.02). In general, trees larger than 0.3 inch (10 cm) in DBH were more likely to support lianas than trees of smaller girth (P<0.03), although this varied with liana and host species. Oriental bittersweet's DBH did not significantly increase with increasing DBH of host trees [88].
Oriental bittersweet assumes a sprawling form on open sites. Sprawling Oriental bittersweet branches may form impenetrable thickets [3,26]. On Naushon Island, Massachusetts, Oriental bittersweet spread horizontally in pastures. Grasses and native shrubs in the pastures, including lowbush blueberry (Vaccinium angustifolium) and black huckleberry (Gaylussacia baccata), were covered to a height of 3 feet (1 m) [126].
On the Pleasant Valley Wildlife Sanctuary, Massachusetts, age of Oriental bittersweet plants was determined 13 years after logging and underbrush removal. Mean basal stem age was 3.4 years (range 1-12 years). Mean plant length was 4.6 feet (11.4 m; range 0.03-6.6 ft (0.01-2 m)). Some Oriental bittersweet plants just outside the Sanctuary (the likely seed sources for the infestation) were 35 years old [143]. In a red maple-American elm forest in West Virginia, Oriental bittersweet individuals (excluding seedlings) ranged from 8 to 23 years old; 15 was the median age [94].
In Pines Hills Campground in southern Illinois, Oriental bittersweet was younger and smaller, but more abundant, in forest-edge than in interior forest communities. Oriental bittersweet averaged 3.3 years of age, 3.9 mm in stem diameter, and 110.5 stems/m² in a forest-edge, white oak-black oak-bitternut hickory community. The canopy was 86% closed. In adjacent interior forests with similar overstory composition, Oriental bittersweet averaged 7.7 years of age, 8.7 mm in stem diameter, and 32.2 stems/m². The interior forest canopy was 96% closed [77].
Oriental bittersweet is native to Korea, China, and Japan [114]. Its southern limit in southeastern Asia is along the Yangtze River watershed (Cheng and Huang 1999 cited in [95]). Oriental bittersweet is nonnative in North America [42,64,70,110,129,166,167,174] and New Zealand [167,173].
In North America, Oriental bittersweet is sporadically distributed from Ontario and Quebec south through the Great Lakes states, New England, and the Southeast to Arkansas, Tennessee, Florida, Louisiana, and the southeastern edge of the Great Plains [24,24,47,70]. It was introduced in the United States around 1860 as an ornamental and for erosion control [131]. It spread to Connecticut by 1916, Massachusetts by 1919, and New Hampshire by 1938 [105]. By 1974, Oriental bittersweet had spread to 33 states [94,120] and was considered invasive in 21 [94]. As of 2011, it was widespread in the Northeast and sporadic [32] but locally dominant [24] farther south [32]. Plants database provides a distributional map of Oriental bittersweet in the United States.
Oriental bittersweet is most common and invasive in New York, coastal Connecticut, and the southern Appalachian Mountains [24]. In 2008, it covered at estimated 8,960 acres (3,630 ha) in forests of the Southeast and South [107]. Using biogeographical models, Leicht [87] predicted that Oriental bittersweet could increase in New England and spread further north. Based on Oriental bittersweet's native range and habitat preferences, others also expect Oriental bittersweet to expand its range in the United States and Canada [27,81].
Given the potential for rapid Oriental bittersweet growth in early postfire environments, prescribed fire alone seems unlikely to control and would probably increase Oriental bittersweet. An invasive plant guide stated that Oriental bittersweet is not a fire hazard, but speculated that Oriental bittersweet is likely to sprout after prescribed fire and that a nutrient flush and increased light availability after fire might promote Oriental bittersweet spread. The authors concluded that prescribed fire was not an option for Oriental bittersweet control [32].
Preventing postfire establishment and spread: More research is needed to determine fire tolerance and postfire response of Oriental bittersweet on specific sites and ecosystems in which it occurs. The Guide to noxious weed prevention practices [160] provides several fire management considerations for weed prevention in general that apply to Oriental bittersweet. Guidelines for determining burn severity, revegetation necessity, and establishing and managing competitive plants are also available [4,43]. See Integrated noxious weed management after wildfires [43] for a more details.
Fire suppression activities may inadvertently promote Oriental bittersweet establishment. On scrub oak sandplains of Martha's Vinyard, Oriental bittersweet established along firelines [121].
Preventing invasive plants from establishing in weed-free burned areas is the most effective and least costly management method. This may be accomplished through early detection and eradication, careful monitoring and follow-up, and limiting dispersal of invasive plant propagules into burned areas. General recommendations for preventing postfire establishment and spread of invasive plants include:
For more detailed information on these topics, see the following publications: [4,11,43,160].
Use of prescribed fire as a control agent: As of early 2011, there was only one study on using fire to control Oriental bittersweet, so possible effects of prescribed fire use in areas with Oriental bittersweet were speculative. Based upon Oriental bittersweet's patterns of regeneration and succession, prescribed fire alone is more likely to increase than control Oriental bittersweet due to postfire sprouting and rapid growth under an open canopy. A Virginia fact sheet noted that merely top-killing Oriental bittersweet, and not killing the roots, results in "vigorous regrowth" [163]. If burning is prescribed to meet management objectives other than control of Oriental bittersweet, burning could be done either very early or very late in the burning season to decrease risk of postfire Oriental bittersweet spread. Because Oriental bittersweet sprouts and establishes from seed, postfire follow-up control, such as grubbing or spraying, and monitoring will be needed (see Control). Either early spring burning or late fall burning, when most associated plant species have gone dormant but Oriental bittersweet is still green, may lower Oriental bittersweet's carbohydrate reserves [132,152]. Polatin's [126] study (see Plant response to fire) suggests that fall prescribed fire is more likely to reduce Oriental bittersweet cover than spring prescribed fire. Small fires and an adaptive approach would allow managers to assess Oriental bittersweet's response over a small area, where it would be relatively easy to monitor Oriental bittersweet's postfire seedling and sprout production.
Altered fuel characteristics: Oriental bittersweet may increase fuel loads and provide ladder fuels, although as of early 2011, studies were lacking on Oriental bittersweet's effects on fuel loads and structure. See Fuels for further details.FIRE REGIMES differ across Oriental bittersweet's North American range. In northeastern maple-birch-beech (Acer-Betula-Fagus) forests, historic fire return intervals were highly variable, depending upon microclimate, topography, and soil. Fires were mostly of mixed severity. Stand-replacing, medium-interval (~ 80-year) fires were most common in forests dominated by birches, while long-interval (≥300 years), mixed-severity or stand-replacing fires occurred in forests dominated by maple and/or beech [33,37,53,138]. Oak-hickory, oak-pine, and pine forests of the Northeast and Southeast had mostly short-return interval, surface fires [151]. Oriental bittersweet was not present in these forests while historic FIRE REGIMES were still operating. See the Fire Regime Table for further information on FIRE REGIMES of vegetation communities in which Oriental bittersweet may occur. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find FIRE REGIMES".
A dearth of fire studies makes it unclear how Oriental bittersweet may affect or alter FIRE REGIMES in plant communities where it is present. Oriental bittersweet's climbing habit can alter forest structure [26,34,93] and may carry fire into the canopies of forests that historically did not experience crown fires. The fire ecology of Oriental bittersweet is poorly understood [132], and research is needed to determine Oriental bittersweet's impact on fire behavior and FIRE REGIMES.
Fire adaptations: Oriental bittersweet sprouts from the root crown and roots [3,26,30,152]. Additionally, it produces abundant, animal-dispersed seed and shows rapid growth on open sites [25,49,62,135,152,171] (see Regeneration Processes). Although there is no literature suggesting that these regeneration strategies evolved in response to fire disturbances in southeastern Asia, sprouting, bird dispersal of off-site seed, and rapid growth would likely favor Oriental bittersweet regeneration in postfire environments of North America.
Plant response to fire: Published accounts of Oriental bittersweet's response to fire were sparse as of this writing (2011); however, since Oriental bittersweet can sprout from the root crown and roots and establishes from animal-dispersed seed following other types of disturbance, it would likely establish from sprouts and/or seed after fire. Dibble and others [21] inferred that species like Oriental bittersweet, which persist in closed canopies and spread after the canopy opens, may spread and become dominant in early postfire environments. Both Oriental bittersweet sprouts and seedlings respond to canopy release with rapid growth [49,104] (see Successional Status and Plant growth). Oriental bittersweet may increase after nutrient flushes (see Disturbance), which often occur after fire [1,2,69]. It may also establish later in postfire succession. In southeastern Connecticut, portions of an old field that was burned and abandoned 40 years prior to the study were dominated by Oriental bittersweet, with Japanese and Morrow's honeysuckle also establishing around postfire year 40 [19].
Seedling establishment: New burns may provide favorable sites for Oriental bittersweet establishment. Field and laboratory studies suggest that open conditions [30,94,117,143] and exposed mineral soil [103,143] favor Oriental bittersweet seedling establishment on mesic sites. Plants with bird-dispersed seed have good opportunities for short- and long-distance seed transport after fire [84]. Birds are known to disperse Oriental bittersweet seeds from nearby seed sources onto old fields and other disturbed sites [34,104,135], so they would likely disperse Oriental bittersweet seed onto burns as well. Long gut-retention times for Oriental bittersweet seed may result in long-distance dispersal by birds [39]. Greenberg and others' [48] germination study suggests that seed scarification by fire or animals does not enhance Oriental bittersweet germination rates.
Because most Oriental bittersweet seed remains viable in the seed bank for only 1 year [30,162], fire that kills a year's cohort of Oriental bittersweet seedlings, and any seeds remaining on plants, would remove most of the Oriental bittersweet seed bank. Even with severe fire, however, it is unlikely that a single fire would kill all Oriental bittersweet seed sources on or near a site. Because Oriental bittersweet is a prolific seed producer, its seed bank can be quickly replenished by on-site, unburned plants or nearby seed sources [7].
Sprouting: Plants that can sprout from the roots, such as Oriental bittersweet (see Vegetative reproduction), can be powerful competitors for space, light, and nutrients in early postfire environments [71,74,99,136]. Roots are less susceptible to fire damage, and often capable of greater carbohydrate storage, than rhizomes and root crowns [73,74]. Although many of Oriental bittersweet's associates in eastern mixed-hardwood forests can sprout from their root crowns (for example, [8,36]), Oriental bittersweet may have a competitive edge over associated species that cannot root sprout. Huebner [60] speculates that growing-season fire may increase Oriental bittersweet abundance by promoting Oriental bittersweet root sprouting.
On Naushon Island, Massachusetts, the effectiveness controlling Oriental bittersweet by mowing followed by either spring or fall prescribed burning was compared with the effectiveness of mowing followed by either 1) spring mowing, 2) fall mowing, 3) spring herbicide (triclopyr), or 4) fall herbicide treatments. The management objective was to reduce encroachment of nonnative Oriental bittersweet, nonnative Scotch broom (Cytisus scoparius), and native common greenbrier (Smilax rotundifolia) onto a remnant little bluestem-indiangrass (Sorghastrum nutans) prairie. Initially, all plots except the untreated controls were mowed to reduced standing woody vegetation. Early-season (spring) plots were mowed in early May, with follow-up spot burning, mowing, or spraying in July. Late-season (fall) plots were mowed in July, with follow-up spot burning, mowing, or spraying in mid-October. Oriental bittersweet cover was less on fall-burned plots than on unburned control plots, although the difference was not statistically significant. Spring prescribed fire had no appreciable effect on Oriental bittersweet cover compared to controls. In both spring and fall treatments, herbicide reduced Oriental bittersweet the most [126].
Mean percent difference (SD) in Oriental bittersweet cover (%) on early- and late-season treatments from cover on control treatments on Naushon Island [126] Treatment Early season Late season Control 6.3 (11.6) 14.6 (6.0) Burn -8.3 (6.7) -25.0 (5.9) Mow -8.3 (9.7) +8.3 (6.8) Herbicide -50.0 (6.4) -72.9 (9.0)Native graminoids had positive responses to fall fire or herbicide application, averaging 20.8% higher cover on burned or sprayed plots than on control plots. Spring burning had no effect on graminoid cover compared to control plots, while early-season herbicide plots averaged 31.3% greater graminoid cover than control plots [126].
Oriental bittersweet may alter fuel structure and loads (review by [21], communication by [133]), although quantitative data of Oriental bittersweet fuel loads were lacking as of early 2011. Oriental bittersweet may act as a ladder fuel by growing up and over supporting shrubs and trees [34,60], which increases the chance that a fire will crown [21]. Oriental bittersweet can also support other lianas and vines [34], possibly enabling other twining species to become ladder fuels as well. It also contributes to understory fuel loads. In total, it contributes substantial biomass to forests of the Northeast [49]. Oriental bittersweet sometimes attains heavy understory cover in hardwood and coniferous communities, such as yellow-poplar, that historically had sparse understories (for example, [124]). Forest understories with Oriental bittersweet or a mix of Oriental bittersweet and other nonnative invasive woody shrubs, such as Japanese honeysuckle and Japanese barberry, can have substantially greater overall cover than uninvaded forests( [19], review by [21]). On opens sites where host plants are scarce, Oriental bittersweet's sprawling habit may also increase fuel continuity and loads over historic levels. Studies are needed on how Oriental bittersweet affects fuel characteristics.
In the laboratory, Oriental bittersweet's mean heat of combustion was near average for northeastern nonnative and native lianas and shrubs [18,20].
In the field, Oriental bittersweet seeds likely require overwintering to germinate. The embryos are dormant, and seeds require stratification for germination ([13,26,48], fact sheet by [24]). Overall germination rate of Oriental bittersweet seed is high [24,152,162], showing 85% (review by [170]) to 95% (fact sheet by [24]) germination in the laboratory.
Mechanical or chemical scarification of the seed is not necessary for germination [30,49,120]; however, germination in the laboratory is delayed or reduced when arils remain attached to the seeds [49]. In a laboratory study, Oriental bittersweet seeds that had either been ingested by birds or had the fruits and arils removed manually showed similar, and higher, germination rates (x=82%) than seeds with intact fruits (x=51%). The authors concluded that although animals aid in seed defleshing and dispersal, gastrointestinal scarification is not needed for germination to occur [48]. Near Asheville, North Carolina, seeds that fell beneath the parent plant, and thus were not ingested by animals, showed reduced germination rates (51%) compared to ingested seeds (82%). However, the authors noted that 51% germination was high enough to substantially contribute to Oriental bittersweet recruitment. Seeds still on the plant in February were damaged and unviable [49].
Light is not required for germination [26,143]. Oriental bittersweet germination occurs under a wide range of light intensities [119]. Greenberg and others [48] found Oriental bittersweet seed germination and seedling survival rates in the greenhouse were similar from 20% photosynthetically active radiation to full sunlight (P=0.05 X² value). They concluded that the ability to germinate over a wide range of light intensities allows Oriental bittersweet to establish under closed canopies [48]. In the greenhouse, Oriental bittersweet germination rates were not significantly different under 20% sunlight to full sunlight, although seedlings with ≥70% full sunlight had more leaves, more stem and root biomass, and longer roots than seedlings in lower light levels [48]. In contrast, Patterson [120] found best germination occurred under low-light conditions in the greenhouse.
Some water-dispersed seed may germinate on floodplains, although seed that floats may be less viable than seed that cannot float. In Connecticut, Oriental bittersweet seed viability was tested for floating vs. sinking seeds. Mean viability was 41% for floating seeds and 88% for sinking seeds [26].
In the United States, Oriental bittersweet grows on woodland [174] and forest [3,94,97,98] edges; in thickets, woodlands, and forests [3,42,110,129,152,166]; and on coastal wetlands, beaches, and saltmarsh edges [24,152]. Oriental bittersweet is common on disturbed sites such as roadsides [3,117,129], logged forests, and old fields [3,129]. It is also common in urban areas, from which it may disperse onto wildlands. Most Oriental bittersweet specimens in West Virginia herbaria had been collected in open forests [59]:
Three studies report site characteristics associated with Oriental bittersweet invasion.
On the Cheat Ranger District of the Monongahela National Forest, West Virginia, site conditions that increased susceptibility of sugar maple forests to invasive species, including Oriental bittersweet, included high overall species richness, north-facing slopes, mesic conditions, and clearcuts (P<0.05 for all variables). Red oak tended to codominate the sugar maple stands with these characteristics [63].
In a study of mixed-oak and yellow-poplar-sweet birch forests on the Bent Creek Experimental Forest, Oriental bittersweet was significantly associated with wind disturbance, topographical features associated with mesic conditions, bare mineral soil, tree harvest, and canopy gaps. Oriental bittersweet was most common at relatively high elevations (x=2,460 feet (749 m)), on steep slopes (x=36% slope), and on concave landforms. It was significantly associated with mesic soils, soils disturbed by animal scarification, and logged sites. Significant stand structure variables for Oriental bittersweet occurrence were canopy gaps, dense midstories, and hardwood overstories dominated by species other than oak. The 2 variables of strongest significance were lack of an oak overstory (P=0.001) and presence of animal-scarified soils (P=0.001). Oriental bittersweet was weakly associated with sites where Hurricane Opal had uprooted trees (P<0.157). McNab and Loftis [103] developed a rapid survey technique and probability-of-occurrence model for hazard rating in areas where Oriental bittersweet is known to occur. Although the study was conducted in western North Carolina, the results may be applicable over a broader area of the Southern Appalachian Mountains with similar environmental conditions [103].
Based on site variables, Pande and others [117,118] provide a model to predict Oriental bittersweet presence across southern Illinois. In their study, best predictors of Oriental bittersweet presence were high elevation, mesic soils, clay soils, proximity to roads, and an overstory other than oak [117,118]. However, Oriental bittersweet is invasive in other oak-hickory forests in southern Illinois [77], Pennsylvania, and New Jersey [61].
Soils: Oriental bittersweet grows on forest, alluvial, and floodplain [129,134,141], and glacial till [50] soils of all textures but of generally acidic pH. Oriental bittersweet typically grows in loam [94,158], sand, and silt [50,134] soils. In New Hampshire, it was negatively associated with soils having high percent clay content (P=0.05) [68]. However, in Giant City Park, Illinois, Oriental bittersweet presence was positively associated with mesic soils with high clay content (x=25% clay, P<0.05) and relatively high pH (x=pH 5.02, P<0.001) [117,118]. Parent materials of soils supporting Oriental bittersweet include granite, sandstone, hornblende, and gneiss. Soil pH is generally moderately to mildly acidic (pH 5.6-6.5) [134], although Oriental bittersweet is reported on gneiss- and schist-derived soils that are <5.5 in pH [103]. Oriental bittersweet occurred on acidic (4.9-5.3 pH) soils in Massachusetts [143], with best establishment and growth on relatively less acidic soils [134]. Transplant experiments conducted 13 years after logging on the Pleasantville Valley Wildlife Sanctuary showed that low pH, high irradiance, and high moisture content were associated with Oriental bittersweet invasion [143].
Oriental bittersweet is most common on mesic soils. It is generally intolerant of saturated or droughty soils [65,143,152,173] but appears tolerant of a range of soil moistures. It may occur on seasonally flooded soils [124] and may establish on some sites despite drought. Oriental bittersweet seedlings in Massachusetts survived a severe summer drought [29] (see Plant growth). More research is needed on moisture requirements for Oriental bittersweet [103].
Oriental bittersweet apparently prefers nutrient-rich soils [5,170]. In hardwood forests of Connecticut, soils supporting Oriental bittersweet had significantly higher potassium, calcium, and magnesium levels than soils without Oriental bittersweet. Nitrogen mineralization and litter decomposition rates were higher on plots with than without Oriental bittersweet (P<0.001 for all variables) [88].
Climate, elevation, and topography: Oriental bittersweet tolerates a wide range of climatic conditions [65,113,120]. It is native to temperate and tropical regions on southeastern Asia [167]. Its elevational range is from sea level to 4,600 feet (0-1,400 m) in the United States [24,103,135]; 1,500 to 7,200 feet (450-2,200 m) elevation in its native range of southeastern Asia (Cheng and Huang 1999 cited in [95]); and from sea level to 1,800 feet (0-540 m) in New Zealand [173]. In Giant City Park, Illinois, Oriental bittersweet presence was positively associated with relatively high-elevation (x=669 feet (204 m)), flat sites (P<0.001 for both variables) [117,118].
Topography varies among sites with Oriental bittersweet, although Oriental bittersweet may be most abundant on mesic depressions or slopes. In Piscataway and Fort Washington National Parks, Maryland, Oriental bittersweet occurs on floodplains, lowlands near the Potomac River, streambanks, stream terraces, and in ravine and upland forests [147]. Along the Blue Ridge Parkway, Oriental bittersweet was more common on relatively moist, north-facing slopes than on dry, south-facing slopes, and occurred up to 6,542 feet (1,994 m) elevation [48]. In a red maple-American elm forest in West Virginia, however, Oriental bittersweet was more common on south- than north-facing slopes [94]. In yellow-poplar/spicebush forests of Inwood Park, Manhattan, Oriental bittersweet was most common on ridgetops (mean density=788 stems/ha) and least common in valleys (36 stems/ha); it was more common on west-facing (589 stems/ha) than east-facing (100 stems/ha) slopes [35].
Impacts: Oriental bittersweet is considered a "severe" pest plant in the Northeast and Southeast [152]. In the Northeast, it is listed as a high threat in deciduous, coniferous, and mixed conifer-deciduous forests, old fields, grasslands, riparian areas, and fresh wetlands; and an unknown threat in tidal wetlands [21]. On the west end of Long Island, for example, Oriental bittersweet was the most abundant nonnative species on Jamaica Bay Wildlife Refuge, where it invaded old fields, thickets, and woodlands [146]. Invasion traits of Oriental bittersweet include:
Reports of Oriental bittersweet's invasiveness vary. Some classify it as invasive [38] to highly invasive [24]. Voss [166] describes it as "sometimes aggressive" when escaped from cultivation in Michigan. On the Energy Oak Ridge National Environmental Research Park, Tennessee, Oriental bittersweet was ranked the 5th most invasive nonnative dicot and the 9th most invasive nonnative plant species overall [22]. In Farmington, Maine, surveyors of invasive nonnatives in mixed hardwood-spruce (Picea spp.) forests ranked Oriental bittersweet as intermediate in abundance, behind Japanese knotweed (Polygonum cuspidatum), Morrow's honeysuckle, Tatarian honeysuckle (Lonicera tatarica), and hybrids of the 2 honeysuckles [6].
The ease of seed dispersal and horticultural interest in Oriental bittersweet in the East and elsewhere in the United States creates a potentially large area for Oriental bittersweet invasion [24]. The southern Appalachians are particularly affected by new invasions [24,81]. In southwestern North Carolina, for example, Oriental bittersweet was one of the most commonly encountered nonnative species, occurring at 53% mean frequency on all of 25 watersheds sampled [80]. Available data and climate models suggest that Oriental bittersweet is likely to benefit from warming temperatures and increasing precipitation in the Northeast, where it is likely to increase and spread northward (review by [27]).
Oriental bittersweet presence may alter soil chemistry, plant succession, and stand structure; threaten native plant diversity; and reduce productivity in silvicultural and agricultural systems.
Effects on succession and stand structure: Oriental bittersweet may outcompete native vegetation for light and modify stand structure, altering historic patterns of plant succession ([9,24,164], review by [152]). Photosynthesis of host and understory plants can be reduced or prevented by Oriental bittersweet. Patterson [120] noted the scarcity of other plant species beneath Oriental bittersweet canopies in Pennsylvania and attributed it to shading by Oriental bittersweet. Oriental bittersweet's growth habit (blanketing and shading out support species) negatively affects the health of host plants and increases continuity of vegetation among forest strata [26,34,48,93] (see Fuels). Twining Oriental bittersweet stems may girdle support vegetation, restricting sap and water flow and damaging or killing host plants. Damaged hosts are at risk for stem breakage and uprooting from ice- and windstorms [24,25,26,93,104,152]. Oriental bittersweet may overtop other plant species in all strata. It may also inhibit or facilitate growth of other lianas. In Connecticut, Oriental bittersweet altered hardwood forest succession by inhibiting reproduction and growth of native shrubs and trees and facilitating growth of fox grape, a late-successional native liana, into the canopy (see Old fields) [34]. Conversely, Oriental bittersweet interfered with growth of native grapes (Vitis spp.) on the Pisgah National Forest, North Carolina [104]. See Successional Status and Stand structure for more information.
Effects on diversity: Oriental bittersweet can displace native species. Its thickets [104] and climbing stems cast too much shade for many native plant species to establish and grow. For example, Oriental bittersweet canopies inhibited establishment of understory spring ephemerals in Illinois [65]. Along the Blue Ridge Parkway in North Carolina, Oriental bittersweet cover was negatively associated with native plant diversity [48]. On Plummer's Island in the Potomac River of Maryland, a 1912 survey documented presence of American bittersweet and common hop (Humulus lupulus), but in 1980, surveyors concluded that those species "appear to have been replaced entirely by the aliens" Oriental bittersweet and Japanese hop (H. japonicus) [141].
Oriental bittersweet is apparently expanding its range at the expense of American bittersweet [127,148,152]. Partially as a consequence of Oriental bittersweet competition, American bittersweet has protection status is several areas [161]. For example, Connecticut lists American bittersweet as a "species of special concern", and Great Smoky Mountains National Park lists American bittersweet as a "nonreproducing rare plant" (Langdon 1993 cited in [24]). The same characteristics that make Oriental bittersweet often preferred over American bittersweet as an ornamental: faster growth, greater fecundity, and a higher tolerance to varying environmental conditions, are the same characteristics that have enabled Oriental bittersweet to become a successful invader [81]. A field study showed Oriental bittersweet increased its photosynthetic rate with increasing light intensity, while American bittersweet's photosynthetic rate tended to saturate under low light conditions [15]. In a common garden study, Oriental bittersweet showed significantly higher photosynthetic rates and faster growth, aboveground biomass gain, and survivorship than American bittersweet in both sun and shade. Oriental bittersweet showed a positive growth response to presence of neighbors, while American bittersweet's response to neighbors was neutral (P=0.05 for all variables) [87]. In the greenhouse, Oriental bittersweet showed increased height, aboveground biomass, and total leaf mass compared to American bittersweet when both species were grown under reduced red:far red light conditions. The authors concluded that Oriental bittersweet's superior ability to grow under these conditions allows it to persist in the understory and "forage" for gaps and sunflecks, whereas American bittersweet's relative inability to gain height and biomass growth under a canopy ensures its decline unless a canopy gap occurs. Further, Oriental bittersweet's ability to detect far red light, which is transmitted and reflected by neighboring plants, may confer ability to "detect" and grow toward neighboring plants that could potentially provide support for Oriental bittersweet's stems [86].
Surveys generally show that Oriental bittersweet is more adaptable and prolific than its native congener. In Connecticut, very wet sites were the only sites where transplanted American bittersweet seedlings outperformed transplanted Oriental bittersweet seedlings (58% vs. 18% mortality for Oriental and American bittersweet, respectively). Oriental bittersweet averaged higher survival (90% vs. 68%) and about 3 times more aboveground biomass (1.93 g vs. 0.67 g) than American bittersweet in low light (≤6.4% transmittance) [90]. A New Jersey study showed a 90% germination rate for first-year, soil-stored Oriental bittersweet seed compared to a 65% germination rate for first-year, soil-stored American bittersweet seed [162]. In a Connecticut study, Dreyer and others [26] found Oriental bittersweet showed significantly higher pollen and seed viability than American bittersweet (P<0.001). While recognizing that many environmental and genetic factors affect seedling establishment, the authors stated that such viability could favor Oriental bittersweet over American bittersweet [26].
American bittersweet is further threatened by potential hybridization and introgression with Oriental bittersweet. Greenhouse studies confirm that the 2 bittersweets are cross-fertile [127,172,176] (see Taxonomy). In a preliminary greenhouse study, interspecific pollination between the bittersweets was more successful than intraspecific pollination, with 1 seedling/flower resulting from hybridization and 0.6 seedling/flower resulting from intraspecific crosses. Although not statistically significant due to small sample sizes [127], these results show the need for field studies documenting the extent of Oriental bittersweet × American bittersweet hybridization.
Effects on silvicultural and agricultural systems: Oriental bittersweet may smother or kill timber trees and understory vegetation [125]. Girdling and stem damage from Oriental bittersweet stems lowers the value of timber trees that host Oriental bittersweet. Where it was present before tree harvest, Oriental bittersweet can rapidly overtake a site after harvest. Its sprouts may overtop understory species and overstory trees. On the Pisgah National Forest, Oriental bittersweet covered sapling-sized hardwood and eastern white pine (Pinus strobus) regeneration on small clearcuts [104]. In a Massachusetts clipping experiment, Oriental bittersweet growth ranged from 6.9 to 15 feet (2.1-4.7 m) in 1 year. In contrast, bigtooth aspen (Populus grandidentata) sprouts grew from 3.0 to 5.9 feet (0.9-1.8 m) in 1 year, and yellow-poplar sprouts averaged 4.6 feet (1.4 m) in 1 year (review by [31]). On the Bent Creek Experimental Forest, a high-quality stand of upland oaks was clearcut in the summer of 1977. Oak site index before harvest was above 80, with a basal overstory area of 120 feet? (11 m²). Preharvest Oriental bittersweet density was 830 seedlings/acre and 27 saplings/acre (seedlings were <0.6 inch (2.0 cm) DBH; saplings were >0.6 inch DBH). Seven years after tree harvest, the canopy was nearly 100% Oriental bittersweet [104].
Oriental bittersweet is an alternate host for Xylella fastidiosa. This bacterium vectors several crop diseases including Pierce's grapevine (Vitis) disease, periwinkle (Vinca) wilt, plum leaf scorch and phony peach (Prunus) disease, and variegated chlorosis (affects several genera including oaks, elms, sycamores, citrus (Citrus), and mulberries (Morus)) [101].
Control: The Southeast Exotic Pest Plant Council [145] recommends that Rank 1 and Rank 2 species such as Oriental bittersweet be controlled and managed in the early stages of infestation whenever possible. Because Oriental bittersweet appears to build only a short-term seed bank [30], there are better opportunities for control and a higher probability of success than if seeds were longer-lived. If on-site plants and nearby seed sources are killed before arils mature, subsequent seedling establishment may primarily come from off-site seed sources, with little seedling emergence from the seed bank [24,30]. Monitoring and early control of new outbreaks can then help control Oriental bittersweet [24,24]. Greenberg and others [48] recommend preventing seed dispersal. This implies treating on- and off-site plants before fruiting, whatever control method is used.
Since Oriental bittersweet resembles the native and rare American bittersweet, it is important to correctly identify Oriental bittersweet before control measures begin [152]. See General Botanical Characteristics for information on identification keys.
Prevention: The most efficient and effective method of managing invasive species such as Oriental bittersweet is to prevent their invasion and spread [140]. Preventing the establishment of nonnative invasive plants in wildlands is achieved by maintaining native communities and surveying, monitoring, and controlling new infestations. Dreyer [24] recommends preventing the introduction of Oriental bittersweet into uninfested areas and making early control of small infestations a priority. Inventories to establish Oriental bittersweet presence and densities are needed before control programs, or any silvicultural treatment that opens the canopy, begin. McNab [102] cautions that if Oriental bittersweet is present in the understory, canopy disturbance will probably stimulate its growth.
Monitoring is an important part of an integrated program for Oriental bittersweet control [143]. Monitoring efforts are best concentrated on the most likely sites of Oriental bittersweet invasion: disturbed soil, roadsides, old fields, woodlands, and waterways. Survey uninvaded sites periodically to detect new invasions [24]. Because Oriental bittersweet retains its leaves longer than most associated native species, its yellow leaves are easy to spot in late fall, even from a distance. Consistent fall monitoring can identify new infestations, allowing managers to implement control programs and prevent new infestations from spreading. Managers in Great Smoky Mountains National Park recommend scouting for infestations every 2 weeks after most native species have dropped their leaves, which is approximately November 10th in the Park [24]. Discouraging nurseries from stocking Oriental bittersweet [55] and encouraging plantings of alternative native ornamentals (see Ornamental and rehabilitation use) can reduce new introductions [55]. The Center for Invasive Plant Management provides an online guide to noxious weed prevention practices.
Monitoring may be more efficient if areas at high risk for Oriental bittersweet invasion are identified. See Site Characteristics for further information on Oriental bittersweet site preferences. For mountainous terrain in the southern the Appalachians, McNab and Loftis [103] describe a rapid survey technique for Oriental bittersweet hazard rating, and they provide a model for estimating probability of Oriental bittersweet occurrence for similar environments based on environmental, competitive, and disturbance factors.
Fire: See Fire Management Considerations for information on using prescribed fire to control Oriental bittersweet.
Cultural control: No information is available on this topic.
Physical or mechanical control: Frequent cutting, mowing, or grubbing helps control Oriental bittersweet. Any portion of stems or roots left on site may sprout [94]; grubbed roots usually sprout unless they are completely removed [23,24,103,152]. Small plants can by hand-pulled, but they need to be moved off site to prevent rooting [168]. Climbing or trailing stems must be cut as close to the root crown as possible (fact sheet by [24]). When grubbing, roots need to be bagged and either removed from the site or allowed to sit in the sun until the bagged plants and seeds have died ([152], fact sheet by [24]). To prevent posttreatment seedling establishment, mechanical treatments are best implemented before Oriental bittersweet is in fruit [152]. Occasional mowing, cutting, or grubbing only encourages root sprouting and is not recommended. Unless the entire root system is completely removed, treatments must be frequent enough to eventually exhaust the underground carbohydrate supply. That may be accomplished by cutting or mowing every 2 weeks [23,24,103,152]; however, that is usually not practical in wildlands.
Biological control: As of early 2011, pathogens from Oriental bittersweet's native range had not been approved for use in the United States [24,94,152]. A leaf spot fungus (Marssonina celastri) causes defoliation of Oriental bittersweet in Korea, where Oriental bittersweet is native [142]. Oriental bittersweet has no known pathogens in North America [152]. This may be a factor in Oriental bittersweet's invasiveness in the United States [117].
Chemical control: Oriental bittersweet can be controlled with herbicides, using either cut-stem or foliar applications. Systemic herbicides (for example, triclopyr or glyphosate) are recommended [24,152].
Effective use of herbicides requires appropriate herbicide concentration, application technique, and timing. For cut-stem treatments, best Oriental bittersweet control occurs when the herbicide is applied soon after stems are cut or mowed [24,152]. Cut the stems about 2 inches (5 cm) above the root crown. A second treatment may be needed to control sprouts [152]. Polatin [126] found mid-October application of triclopyr gave better control of Oriental bittersweet than spring application. Herbicide applications in early spring, before native herbs have emerged, or in late fall when natives are dormant but Oriental bittersweet is still green, can minimize effects to nontarget plants [152].
In red pine (Pinus resinosa) forests in Connecticut, late-summer (17-18 September) herbicide treatments gave fair to good control of Oriental bittersweet. Fourteen treatments involving four different herbicides, used alone or in combination, were used, with various application rates. One year after treatments, imazapyr and triclopyr gave best results with low concentrations. See Ahrens [3] for details of herbicide combinations, concentrations, and other treatment results.
See these sources: [12,17,24,32,94,126,152] for more information on chemical control of Oriental bittersweet.
Herbicides may provide initial control of a new invasion or a severe infestation, but used alone, they are rarely a complete or long-term solution to invasive species management [12]. Herbicides are most effective on large infestations when incorporated into long-term management plans that include replacement of weeds with desirable species, careful land use management, and prevention of new infestations. Control with herbicides is temporary, as it does not change the conditions that allowed the invasion to occur (for example, [177]). See The Nature Conservancy's Weed control methods handbook [159] for considerations on the use of herbicides in Natural Areas and detailed information on specific chemicals.
Integrated management: No single treatment provides effective, long-term control of Oriental bittersweet. Integrated management includes early detection, assessment, and containment of infestations before they spread. Factors to be addressed before a management decision is made also include assessment of nontarget vegetation, soil types, climatic conditions, important water resources, and an evaluation of the benefits and limitations of all control methods [112]. Hobbs and Humphries [57] advocate an integrated approach to the management of plant invasions that includes "a focus on the invaded system and its management, rather than on the invader" and "identification of the causal factors enhancing ecosystem invasibility" as an effective approach to controlling invasive species. This emphasizes removing the ecological stressors that may be underlying the causes of invasion rather than focusing on direct control of invasive species [57].
Few studies to date (2011) investigated using multiple control methods for managing Oriental bittersweet. Compared with mowing alone or mowing and prescribed fire combined, Polatin [126] found a combination of mowing and triclopyr was "by far the most effective treatment for controlling bittersweet and allowing for grass establishment". See Plant response to fire for details of this study. Hutchison [65] recommends either grubbing or a combination of cutting and herbicide treatment. When practical, he recommends uprooting and removing individual Oriental bittersweet stems from infested sites. In other situations he recommends hand cutting after the first killing frost, then spot-treating cut stems with glyphosate. To maintain control, he advocates immediately pulling and removing invading individuals off site [65].Birds and mammals eat Oriental bittersweet arils. Birds that consume and disperse Oriental bittersweet arils include black-capped chickadees, eastern bluebirds, northern mockingbirds, European starlings, blue jays, northern bobwhites, ruffed grouse, ring-necked pheasants, [24,123,150,170], and wild turkeys (Poole 2005 personal communication in [21]). However, it is unclear how important Oriental bittersweet arils are as an avian food source [77]. Frugivorous mammals that eat the arils include fox squirrels and eastern cottontails (review by [170]). White-tailed deer may browse the foliage, although the foliage may not be preferred. In Rock Creek Park, Washington DC, Oriental bittersweet was more frequent in exclosure plots than in control plots that were accessible to white-tailed deer [137].
Palatability and nutritional value: Oriental bittersweet browse is apparently unpalatable to herbivores. Although cattle, white-tailed deer, and lagomorphs browse Oriental bittersweet's congener, American bittersweet [16], browsing animals do not similarly utilize Oriental bittersweet [23].
Oriental bittersweet arils are nutritious. Arils collected on Block Island, Rhode Island had higher protein (8.6% dry weight) and carbohydrate (89.1% dry weight) content than fruits of 8 associated woody species [144]. Fresh Oriental bittersweet arils are 71% water [39]; seed oil content is about 50% [175]. The arils may be toxic to humans (review by [170]).
Cover value: Information on wildlife use of Oriental bittersweet for cover was sparse as of early 2011. Because Oriental bittersweet can alter forest structure, it probably favors thicket- and understory-dwelling animals at the expense of animals using other strata. On a Nature Conservancy Preserve on Long Island Sound, Connecticut, Oriental bittersweet threatened sand dunes providing nesting areas for the piping plover, a state-threatened bird. Managers were concerned that Oriental bittersweet would either spread onto and overtake nesting areas or alter dune erosion and formation dynamics (Lapin 1992 cited in [24]). In an American beech-yellow-poplar forest in Delaware, veeries selected nesting areas with nonnative vegetation cover significantly more than areas with native vegetation cover (P=0.05). Oriental bittersweet was among the nonnative vegetation providing cover near nests, although multiflora rose was usually selected as the actual nest shrub [56].
In the eastern United States, Oriental bittersweet is most abundant in mesic, mixed-hardwood forests and forest edges [72,103,134,169]. It may also be common in coniferous forests [49,103,115] and in woodland, (fact sheets by [24,152]), shrubland [156,165], old field [134], duneland, coastal beach (fact sheet by [105]), tidal freshwater [85], and saltmarsh communities (fact sheet by [105]).
Great Lakes states:
In the Great Lakes states, Oriental bittersweet occurs in mixed-hardwood, pine (Pinus spp.), and prairie or prairie-edge communities. In Giant City Park of Carbondale County, Illinois, Oriental bittersweet presence was positively associated with that of yellow-poplar (Liriodendron tulipifera) and negatively associated with that of oaks (Quercus spp.) (P<0.001 for both variables). It was present in but not significantly associated with pine communities and was absent from silver maple (Acer saccharinum) and baldcypress (Taxodium distichum) swamps [117,118]. Although Oriental bittersweet sometimes avoids oak communities, in southern Illinois it was common in mixed-oak-bitternut hickory (Carya cordiformis) as well as mixed-deciduous forests [77], and in Delaware Gap National Recreation Area of Pennsylvania and New Jersey, Oriental bittersweet was found only in oak-hickory forests [61]. In Ohio, Oriental bittersweet was a component of wetland prairies dominated by either narrowleaved mountainmint (Pycnanthemum tenuifolium) or Dudley's rush (Juncus dudleyi) [40].
New England:
Oriental bittersweet is documented in mixed-hardwood, conifer, shrubland, and old-field communities of New England. In coastal southern New England, Oriental bittersweet was more common in pitch pine/wavy hairgrass (P. rigida/Deschampsia flexulosa) forests (2.2% mean Oriental bittersweet cover) than in bear oak/northern bayberry-rose (Quercus ilicifolia-Myrica pennsylvanica-Rosa spp.) shrublands (1.4% Oriental bittersweet cover) or on open sites that had <25% woody plant cover (0.6% Oriental bittersweet cover). It did not occur in heathlands (Ericaceae) [165]. In Amherst, Massachusetts, Oriental bittersweet occurred in the understory of a northern red oak-hickory-red maple (Quercus rubra-Carya spp.-Acer rubrum) forest. Native and nonnative honeysuckles (Lonicera spp.) dominated the shrub layer [30]. On riparian floodplain forests of Massachusetts, Oriental bittersweet occurred in the understory of a sugar maple-eastern cottonwood (Populus deltoides subsp. deltoides) forest. Sycamore (Platanus occidentalis) and white ash (Fraxinus americana) were occasional in the canopy. Slippery elm (Ulmus rubra), hackberry (Celtis occidentalis), and boxelder (A. negundo) dominated in the
subcanopy. Other riparian species occurring with Oriental bittersweet in the understory included staghorn sumac (Rhus typhina), nonnative multiflora rose (Rosa multiflora), and nonnative Japanese barberry (Berberis thunbergii), with Japanese barberry most common. Ostrich fern (Matteuccia struthiopteris) dominated the herb layer [72]. In central and western Massachusetts, Oriental bittersweet, multiflora rose, and Japanese barberry were positively associated with one another (P<0.001) [100]. Oriental bittersweet occurred and sometimes codominated in Allegheny blackberry-porcelainberry (Rubus allegheniensis-Ampelopsis brevipedunculata) shrublands of Rock Creek National Park, Washington, DC [156].
In the Pennyback Wilderness of southeastern Pennsylvania, Oriental bittersweet occurred in mixed-mesophytic woodland and forest, riparian, and old-field communities.
American beech (Fagus grandifolia), oaks, yellow-poplar, white ash, red maple, and black walnut (Juglans nigra) dominated woodland and forest overstories. Flowering dogwood (Cornus florida), black cherry (Prunus serotina), sweet cherry (P. avium), poison-ivy (Toxicodendron radicans), Canadian woodnettle (Laportea canadensis), goldenrods (Solidago spp.), and nonnative Japanese stiltgrass (Microstegium vimineum) were common understory components. Boxelder, sycamore, green ash (F. pennsylvanica), and silver maple dominated riparian zones. Blackberries (Rubus spp.) dominated old fields. Little bluestem (Schizachyrium scoparium), milkweeds (Asclepias spp.), and Indianhemp (Apocynum cannabinum) were common old-field components. Nonnative Japanese honeysuckle (L. japonica) associated with Oriental bittersweet in each of
the 4 community types [134].
Southeast:
In the Southeast, Oriental bittersweet occurs mostly in mixed-hardwood and old-field communities. On the George Washington Memorial Parkway in Virginia, Oriental bittersweet occurred on the edges of late-successional oak-hickory forests. White oak (Q. alba), scarlet oak (Q. coccinea), chestnut oak (Q. prinus), pignut hickory (Carya glabra), and mockernut hickory (C. tomentosa) dominated interior forest overstories. Forest-edge communities were a mix of nonnative and native lianas and herbs including Oriental bittersweet, Japanese honeysuckle, summer grape (Vitis aestivalis), riverbank grape (V. riparia), white clover (Trifolium repens), Kentucky bluegrass (Poa pratensis), common velvetgrass (Holcus lanatus), and broomsedge bluestem (Andropogon virginicus) [169].
On the Bent Creek Experimental Forest near Asheville, North Carolina, Oriental bittersweet occurred in the understory of a mixed-hardwood forest. Yellow-poplar and sweet birch (Betula lenta) dominated on mesic sites, where Oriental bittersweet was most common. Oriental bittersweet was less common on dry sites where scarlet, chestnut, and black (Q. velutina) oak mixed with occasional shortleaf pine (Pinus echinata). Red maple, hickories, and white oak were scattered throughout the mixed-hardwood community [49,103]. Midstory species included red maple, sourwood (Oxydendrum arboreum), and flowering dogwood. Rosebay (Rhododendron maximum), blueberries (Vaccinium spp.), and huckleberries (Gaylussacia spp.) occurred in the shrub layer. Oriental bittersweet did not associate with mountain-laurel (Kalmia latifolia), which was common on the Experimental Forest but tended to occupy relatively dry soils [103]. Another survey in Bent Creek Experimental Forest found Oriental bittersweet was mostly associated with yellow-poplar forests that had succeeded from agricultural fields. Oriental bittersweet was less common in mixed-oak forests, which had no known history of agricultural use [79].
English-language literature on Asian plants communities with Oriental bittersweet's was scant as of this writing (2010). Pande [117] reported that Oriental bittersweet is not considered a forest species in its native Asia. In Japan, Oriental bittersweet occurs in lowland and mountainous thickets and on grassy slopes [114].
Medicine and other products: Oriental bittersweet is an Asian folk medicine used for treating rheumatoid arthritis and bacterial infections. Medical and pharmacological studies show that Oriental bittersweet derivatives have antitumor, antiinflammatory, antioxidant, antibacterial, and insecticidal properties [66,67,108]. One Oriental bittersweet derivative shows ability to reverse multidrug resistance of cancer cells to cancer-treatment drugs [75,76].
Oriental bittersweet bark is used as a fine fiber in China [175]. Enzymes in Oriental bittersweet leaves clot milk. These leaf extracts may provide an alternative to calf rennet enzymes used in making cheese [116].
Ornamental and rehabilitation: In the United States, Oriental bittersweet is commercially available and widely planted and harvested as an ornamental [105]. Wreaths and other ornaments are made from fruiting stems; Oriental bittersweet seeds may disperse if these ornaments are discarded on favorable germination sites. Oriental bittersweet was once widely planted in highway and "conservation" plantings, but it is not currently recommended for wildland plantings [51,54,102].
Alternative native lianas recommended for ornamental and restoration plantings include American bittersweet, trumpet honeysuckle (Lonicera sempervirens), trumpet-creeper (Campsis radicans), yellow passionflower (Passiflora lutea), pipevine (Aristolochia macrophylla), and American wisteria (Wisteria frutescens) [152]. For wildlife plantings, American bittersweet arils can provide food for frugivorous animals [102]. Oriental bittersweet is sometimes mistaken for, mislabeled, and/or planted as American bittersweet in wildlife cover and erosion projects. Correct identification of American bittersweet is needed to meet restoration goals [109].
Oriental bittersweet shows rapid growth under partial to full sun [3,102], although seedlings grow slower in low than in high light [49,119]. In Massachusetts, artificially shaded Oriental bittersweet transplants showed greatest aboveground biomass gain under 28% of full sunlight. Mean aboveground biomass 1 year after transplanting was significantly different (P=0.002) under 100% full sunlight (0.9 g), 28% of full sunlight (14.4 g), and 2% of full sunlight (0.3 g) [29]. Oriental bittersweet growing in partial to full sun can overtop 3- to 7-foot (1-2 m) tall associated vegetation after one growing season [31]. On mesic sites in full sunlight, Oriental bittersweet may grow 10 to 12 feet (3-3.7 m)/year [102]. In Connecticut, Oriental bittersweet grew 10 feet (3 m)/year [120]. The Tennessee Exotic Plant Pest Council [154] reported annual growth rates of 1 to 12 feet (0.3-3.0 m) in the first 7 years after establishment, with little growth thereafter. Oriental bittersweet may persist in a densely shaded understory for many years, then respond with rapid growth when disturbance opens the canopy [102,120].
Besides having both shade and sun tolerance, other factors contributing to Oriental bittersweet's often rapid growth include tolerance to varying soil moisture and ability to form mycorrhizal associations. In Connecticut, transplanted Oriental bittersweet seedlings showed low morality and good height and biomass gain across a wide range of soil moisture and light conditions [89]. See Effects on diversity for further information on this study and Soils for more information on Oriental bittersweet soil tolerances. In greenhouse studies, Oriental bittersweet with their roots infected with mycorrhizae grew taller than plants with uninfected roots when phosphorus was limiting (P≤0.05). When phosphorus was added to the soil, mycorrhizae appeared to limit Oriental bittersweet growth [91]. Oriental bittersweet has shown faster growth rates than associated native lianas. In Michigan, Oriental bittersweet plants were younger than native riverbank grapes (x=3.7 vs. 4.2 years, respectively) but stems were 8% thicker [157].
Clonal growth can result in large patches of Oriental bittersweet that originated from a few seedlings (fact sheet by [24]). Increased seedling vigor (biomass and growth rates) under high light intensity in the greenhouse and higher cover of Oriental bittersweet on open sites led researchers to suggest that canopy disturbance aids vegetative spread of established seedlings [48]. In Connecticut, Oriental bittersweet cover on 0.1 acre (0.06 ha) increased from 5% to 100% within 5 years [120].
A study by Ellsworth [29] illustrates many Oriental bittersweet characteristics that enhance its invasiveness: abundant seed production, high rates of germination and seedling establishment, and tolerance to a wide range of light intensities. The study included field, shadehouse, and greenhouse experiments. Across 15 sites in Massachusetts, Oriental bittersweet seed rain averaged 168 seeds/m². Seed rain was highly variable, however, ranging from 13 to 826 seeds/m². In the greenhouse, germination averaged 68%, and emergence averaged 107 seedlings/m². In the field, emergence averaged 1 seedling/m². In the shadehouse, litter biomass greater than 4 Mg/ha reduced seedling emergence. Survival of Oriental bittersweet seedlings in the field varied and was not associated with Oriental bittersweet seedling density. Survival averaged 16.8% by August; summer weather conditions were "extremely dry". In the field and shadehouse, light intensities of 100%, 28%, and 5% of full sunlight had no significant effect on Oriental bittersweet survival or growth in the 1st growing season. In the 2nd growing season, survivorship was similar (68%) under all light intensities, but seedlings in 28% sunlight had greater total stem length (all stems combined: 15.1 feet (4.6 m)) than seedlings in full sun (7 feet (2 m)) or 5% sun (0.7 feet (0.2 m)). The author predicted that given partially shaded to open conditions, seedlings surviving their 1st year would have high survivorship thereafter. He suggested that given Oriental bittersweet's high seedling survivorship in deep shade and growth plasticity under variable light intensities, intact forests are vulnerable to Oriental bittersweet invasion. He predicted that a density of 12 seedlings/m² is enough to establish a new Oriental bittersweet population on many sites [29].
Oriental bittersweet regenerates by sprouting and from seed. Its invasiveness is due, in part, to its superior ability to establish from both sprouts and seeds compared to most native lianas and other associated native woody species [49,96,106,120,129,143]. Based on Oriental bittersweet's ability to spread from both root sprouts and bird-dispersed seed and its use of multiple breeding systems (see the Regeneration Processes links above), Huebner [62] suggested that Oriental bittersweet may become the fastest spreading invasive species among Oriental bittersweet, Japanese stiltgrass, garlic mustard (Alliaria officinalis), tree-of-heaven (Ailanthus altissima), and European buckthorn (Rhamnus cathartica) [62].
A comprehensive study of Oriental bittersweet regeneration [29] is reviewed in Plant growth.
Oriental bittersweet has a short-lived soil seed bank. Field [29], and laboratory [30] studies suggest Oriental bittersweet seed does not remain viable for more than one growing season, although some managers report that soil-stored Oriental bittersweet seed remains viable for several years [7,24]. The small portion of viable seed remaining in seed banks for more than 1 year contributes little to Oriental bittersweet regeneration [30,78]. A 3-year field study in New Jersey showed a 90% establishment rate of Oriental bittersweet seed the 1st spring after a winter planting. There was no "appreciable" Oriental bittersweet germination in the 2nd or 3rd years of the study [162]. In a 2-year study in an Oriental bittersweet-infested Massachusetts field, Oriental bittersweet seedling recruitment was measured after Oriental bittersweet seed was hand-sown onto study plots. Density of Oriental bittersweet seedling recruitment closely matched the density of seeds sown. Seed rain ranged from 14 seeds/m² to 826 seeds/m² (x=168 seeds/m²). Oriental bittersweet seedling emergence from the seed bank averaged 0.9 seedling/m?. All seedlings emerged in the 1st year of the study, with seedling recruitment ranging from 11 seedlings/m? to 532 seedlings/m? (x=107 seedlings/m?) [30].
In a green ash-yellow-poplar forest near Philadelphia, Pennsylvania, Oriental bittersweet seedlings emerged from the seed bank following herbicide treatment of Japanese stiltgrass. Oriental bittersweet was a common component of the aboveground vegetation before spraying [46].
Field workers in Great Smoky National Park reported substantial Oriental bittersweet seedling recruitment during the first 6 years of a grubbing and herbicide control program involving "complete removal and rootkill" of Oriental bittersweet (Langon 1993 cited in [24]), suggesting that either the seedlings originated from the seed bank or there was an off-site seed source. In a fact sheet, Dreyer [24] noted that because Oriental bittersweet is such a prolific seed producer, its seed bank is quickly replenished when seed sources remain on site or nearby.
Animals, water, and humans disperse Oriental bittersweet seed [26,105,129]. The seed disperses after the 3-valved capsules split open and expose the arils [129]. The brightly colored, fleshy arils attract birds and small mammals, which disperse most of the seed after ingesting the arils [49,96,106,120,143]. The fruits can float if they fall into water [105]. Undispersed seed falls under or near parent plants. Along the Blue Ridge Parkway, North Carolina, 24% of Oriental bittersweet fruits fell to the ground [48].
Frugivorous birds are probably most important for Oriental bittersweet seed dispersal because they are highly mobile, travel in flocks, and often eat "voraciously" [25,49,171]. Northern flickers, yellow-rumpled warblers, American robins and other thrushes (Turdidae), mockingbirds and catbirds (Mimidae), and European starlings and mynas (Sturnidae) are the primary Oriental bittersweet seed dispersers [171]. In an oak forest in North Carolina, small animals removed 75% of the total Oriental bittersweet seed crop [49]. Near Asheville, North Carolina, birds and small mammals dispersed Oriental bittersweet seeds in "large numbers". Still, more than 80% of Oriental bittersweet arils remained on the parent plant until December, and >50% remained until mid-January [49]. Birds likely disperse Oriental bittersweet seeds where they perch. In central Japan, where Oriental bittersweet is native, Oriental bittersweet seed rain was densest under the liana Smilax china (10 seeds/1.5 m²) and the shrub Neolitsea sericea (8 seeds/1.5 m²), both of which were used as bird perches [153].
Seed dispersal by birds is an important factor in Oriental bittersweet's ability to rapidly colonize a site. Long-distance dispersal of seed by birds may promote faster rates of Oriental bittersweet establishment on new sites compared to plant species without bird-mediated seed dispersal, particularly late-successional herbs with seeds that are primarily dispersed by ants [97]. Oriental bittersweet seedlings were noted the first year following restoration plantings in a landfill on Staten Island, New York. Minimum mean travel distance to the nearest Oriental bittersweet seed source was 430 feet (131 m); the authors surmised that birds dispersed Oriental bittersweet seed onto the landfill [135]. Long gut-retention times may result in very long Oriental bittersweet dispersal distances when birds migrate. In Japan, Oriental bittersweet seed remained in the digestive tracts of brown-eared bulbuls (Hypsipetes amaurotis, a native Japanese passerine) for 14 to 42 days (x=27 days). That was one of the longest retention times recorded among 16 bird-dispersed plant species [39].
Roads may act as corridors for seed dispersal. Oriental bittersweet is common along roadsides [117], especially interstate highways in New England [26]. It has been used in roadside plantings in the Northeast. Humans using fruiting branches for ornaments may disperse seeds when collecting or disposing of the branches [24,26]. People can facilitate animal dispersal of Oriental bittersweet seed by planting Oriental bittersweet as an ornamental [25].
Oriental bittersweet seedling emergence is generally high [104,120], but survivorship may vary greatly depending upon field conditions and population differences. Survivorship of field-germinated seedlings in Massachusetts ranged from 0% to 88% (x=17%) [30]. Patterson [120] reported densities of 60 emergents/m? for an Oriental bittersweet population in Connecticut; however, seedling density declined over the growing season. He attributed seedling mortality to drought [120]. In the laboratory, seedling emergence rate differed significantly among 2 Oriental bittersweet populations in Connecticut (P<0.001). Percent emergence after 21 days was 59% and 82% [26].
Oriental bittersweet frequently establishes along fencelines and other sites where birds perch and defecate the seeds [44,45,104,104].
Thick litter may retard Oriental bittersweet seedling emergence. A study on the Bent Creek Experimental Forest found Oriental bittersweet was significantly less abundant in oak communities than in yellow-poplar-sweet birch communities (P<0.005) [103], which might have been partially due to differences in litter depth. A survey in North Carolina found Oriental bittersweet was associated with yellow-poplar forests that had succeeded from agricultural fields, while Oriental bittersweet was less common in mixed-oak forests. The author concluded that thinner leaf litter layers and moister soils in yellow-poplar forests favored Oriental bittersweet germination, seedling establishment, and growth. Survivorship was greater on plots with thin litter (1 kg litter/m²) than with thick (3 kg litter/m²) or no litter (P≤0.01) [79]. A greenhouse study showed that intact oak litter physically impeded Oriental bittersweet seedling emergence, although hypocotyls grew sideways as much as 4 inches (9 cm) to find a point of reduced litter (in this case, the pot edge), and then emerged. Seedlings in deep litter tended to allocate more growth to hypocotyls, while seedlings in shallow litter tended to allocate more growth to cotyledons. The authors concluded that Oriental bittersweet seedlings would probably find litter patches thin enough for emergence in all but the deepest oak litter, but pine litter may be more conducive to Oriental bittersweet establishment than oak litter [30]. A shadehouse experiment showed mixed-deciduous litter loads above 3 Mg/ha lowered Oriental bittersweet seedling emergence (P<0.05), but lesser loads or fine-ground litter had no significant effect on Oriental bittersweet emergence. The author suggested that litter disturbance likely favors Oriental bittersweet establishment [29].
Oriental bittersweet is sun and shade tolerant and may occur in all stages of succession.
Light tolerance: Oriental bittersweet is shade [31,49] and light tolerant at all life stages. In hardwood forests of Connecticut, canopy closure typically ranged from 76% to 100% on sites with Oriental bittersweet [88]. In the greenhouse, varying light intensities did not produce significant differences in either the number of days Oriental bittersweet took to germinate, its germination rate, root:shoot weight, or its root:shoot length. However, seedlings exposed to 70% or more of full sunlight tended to produce more leaves, heavier leaves, and longer, heavier roots than more shaded seedlings [49]. In a Massachusetts field, artificially shaded Oriental bittersweet transplants showed good survivorship over a large range of sunlight intensities. Survivorship was best with moderate shade [31].
Mean (SD) Oriental bittersweet survivorship from midsummer transplanting in 2000 to field measurements in fall 2001 [31] Treatment (% sun) Transplant survival (%) Winter survival (%) Growing season survival (%) Cumulative survival after 1 year (%) 100 96 (3) 49 (21)b* 90 (9)ab 43 (20) 28 87 (19) 84 (12)a 96 (4)a 70 (21) 2 94(7) 60 (12)ab 76 (5)b 43 (11) P value not significant 0.031 0.029 not significant *Within columns, means with the same letter are not significantly different among treatments (P<0.05).Oriental bittersweet employs what Greenberg and others [49] call a "sit and wait" strategy: it can establish, grow slowly, and persist in late-seral, closed-canopy communities. When disturbance opens the canopy, it typically responds with rapid growth [49,104]. In the greenhouse, Patterson [120] found Oriental bittersweet seedlings grown under low light nearly doubled their photosynthetic rate after 8 days of exposure to high light. Oriental bittersweet's ability to establish in low light and then climb towards higher light intensity enables it to shade out, overtop, and eventually kill supporting vegetation [3,26]. When dense, Oriental bittersweet plants may replace the successional subcanopy [34] or the canopy [104].
Disturbance: Disturbance increases the likelihood of successful Oriental bittersweet establishment [30]. Field studies show Oriental bittersweet is most common near roads [117,143] and on sites disturbed by logging, windthrow, animal foraging [103,143], disease, or insect outbreaks [115]. Oriental bittersweet often establishes on roads, then spreads to adjacent forests [94], reviews by [14,27], [105]. In Giant City State Park, Illinois, for example, Oriental bittersweet presence was positively correlated with proximity to roads (P<0.05) [117]. Along the Blue Ridge Parkway in North Carolina, however, Oriental bittersweet cover and density were unrelated to distance from the roadside, although Oriental bittersweet plants were taller along the road than in interior forests [48]. Oriental bittersweet was common in eastern hemlock (Tsuga canadensis) forests in Connecticut following deforestation (≥50% foliar loss) by hemlock woolly adelgids [115].
On the Pleasantville Valley Wildlife Sanctuary, Massachusetts, Oriental bittersweet density was correlated with level of past site disturbance. Sites with a known history of farming or logging tended to have moderate to heavy infestations, with heaviest infestations on logging roads (P<0.005). On logged sites, plots where horse logging had been conducted were less infested than plots where mechanized equipment was used. Oriental bittersweet did not occur on undisturbed plots. Pre- and postlogging surveys suggest that Oriental bittersweet seedling establishment on logged plots occurred 2 or more years after logging ceased. The authors speculated that this establishment lag was due to reluctance of bird dispersers to forage or perch in exposed openings and/or initial colonization by other plant species, which created favorable, mesic conditions that promoted Oriental bittersweet germination and establishment. Oriental bittersweet was positively correlated with percent exposed mineral soil (P<0.025) and sites with relatively less acidic soil (P=0.01), a combination of which was most common on logging roads and least common on undisturbed sites. Although Oriental bittersweet did not occur on undisturbed plots, small but apparently healthy Oriental bittersweet seedlings were present in late-successional deciduous and coniferous forests elsewhere on the Sanctuary, growing through an intact litter layer on a well-shaded forest floor [143].
An invasive plant guide reports that soil disturbance promotes Oriental bittersweet spread [32]. Nutrient flushes, which are often associated with fire and other soil disturbances, may encourage establishment of Oriental bittersweet and other invasive species. In Massachusetts, a black oak-white oak/black huckleberry-low sweet blueberry forest irrigated with nitrogen-enriched wastewater was initially invaded with old-field herbs including pokeweed (Phytolacca americana) and tangled bindweed (Polygonum concolculus). Pokeweed was still abundant after 9 years of wastewater irrigation, but Oriental bittersweet had successionally replaced tangled bindweed. Compared to control plots, bittersweet nightshade (Solanum dulcamara), Tatarian honeysuckle (Lonicera tatarica), Virginia creeper (Parthenocissus quinquefolia), and poison-ivy were also more abundant after 9 years of wastewater fertilization [69].
Land use history and soil chemistry can affect invasibility of a site for Oriental bittersweet and other nonnative plant species. In central Connecticut, Oriental bittersweet was most frequent in recently abandoned (5-year-old) fields (77% frequency), reforested old fields (43%), and sites undergoing residential or commercial development (39%) (P<0.001) [111]. In northeastern Connecticut and southern Massachusetts, land use history was the greatest single predictor of site invasibility. Oriental bittersweet was most abundant on former (1934) residential areas (20% Oriental bittersweet cover, 33% frequency) and old fields (0.46% cover, 28% frequency). In general, Oriental bittersweet cover and frequency were greatest in populated areas (>8 houses/km; 7.63% cover, 50.0% frequency) and least in remote areas (1-2 houses/km; 0.02% cover, 1.7% frequency). Oriental bittersweet was detected most often near paved roads (26%-46% of total invaded plots), with frequency decreasing along dirt roads (7%-39%), trails (0-30%), and forest interiors (1%-17%). Oriental bittersweet cover increased with road size [92]. In central and western Massachusetts, the carbon:nitrogen ratio was the strongest site factor correlated with Oriental bittersweet presence (P<0.001). Forest interiors were also negatively associated with Oriental bittersweet presence (P<0.07) [100].
Forests: Although it occurs in all stages of forest succession, Oriental bittersweet is most common in early-seral deciduous forests and on forest edges ([94], reviews by [14,27], [105]). Oriental bittersweet colonizes forest edges and canopy gaps [27,31] but also grows in very low light conditions such as those found in interior, closed-canopy forests [86]. It may slow or alter succession in young, developing tree stands [125]. After establishing on woodland and forest ecotones in Delaware and Pennsylvania, Oriental bittersweet invaded oak-hickory forests in both second-growth and closed-canopy successional stages [97,98]. Oriental bittersweet was found in communities representing all stages of succession (floodplain riparian forest and old field to mature forest) in mesophytic mixed-oak (Quercus spp.)-American beech and riparian communities of southeastern Pennsylvania. It was most frequent in early and late stages (old fields and forests) [134].
Oriental bittersweet frequency by successional stage and community type in Pennsylvania [134] Floodplain riparian Old field Thicket Woodland Forest 24% 41% 37% 26% 39%Oriental bittersweet sometimes invades mature and old-growth forests [97,98]. Within late-successional forests, however, it may be most common in gaps or where the canopy is relatively thin. In West Virginia, Oriental bittersweet seedlings were more abundant in red maple-America elm forest interiors than on forest edges. Oriental bittersweet cover, however, was negatively associated with tree and shrub cover (P<0.007) [94], suggesting that within forest interiors, Oriental bittersweet establishment was best in openings. Mean Oriental bittersweet seedling density in interior forest sites was 286.9 seedlings/m² compared to 186.3 seedlings/m² on forest edges. The oldest Oriental bittersweet plants were closest to forest edges [94], so Oriental bittersweet probably spread from forest edges to the interior forest. Oriental bittersweet presence was negatively correlated with forest cover in yellow-poplar and mixed-oak forests on the Bent Creek Experimental Forest [79] and 25 watersheds at other locations in southwestern North Carolina [80].
Old fields: Oriental bittersweet occurs in all stages of old-field succession [3,152,158] and may change old-field successional pathways. In southeastern Connecticut, old-field vegetation surrounded by a late-successional oak-hickory forest was measured approximately every 10 years from 1954 to 1992. The old field burned just prior to the 1954 survey. In 1954, field cover was primarily Kentucky bluegrass (Poa pratensis), redtop (Agrostis gigantea), and wrinkleleaf goldenrod (Solidago rugosa). Allegheny blackberry (Rubus allegheniensis) and northern bayberry (Morella pennsylvanica) had scattered occurrence. By 1960, black cherry (Prunus serotina) and black oak had established. By the 1970s, a thicket of shrubs and trees had developed; important new woody species included common greenbrier (Smilax rotundifolia) and flameleaf sumac (Rhus copallinum). Nonnative Oriental bittersweet, Morrow's honeysuckle, and multiflora rose and native Virginia creeper were noted for the first time. By the early 1980s, 2 distinct plant communities were described. The eastern portion of the field was a young hardwood forest dominated by black cherry, red maple, and black tupelo (Nyssa sylvatica). Southern arrowwood (Viburnum dentatum), Morrow's honeysuckle, and common greenbrier dominated the midstory. Woody vines <2.0 feet (0.5 m) tall, including Oriental bittersweet, Virginia creeper, and poison-ivy, dominated the lowest strata. The western portion of the old field was an "almost impenetrable" woody vine community dominated by Oriental bittersweet. Fox grape (Vitis labrusca) was increasing in cover and spreading into the canopy by using Oriental bittersweet for support. Scattered woody species on the western field were covered by nonnative and native lianas. Host trees were suffering branch breakage and low reproduction; shrubs were suppressed or killed. The authors speculated that the initial Oriental bittersweet invasion was facilitated by an Oriental bittersweet plant at the edge of the field, which provided perching support and arils (with seeds) for frugivorous birds. This field-edge Oriental bittersweet plant was 2 inches (5 cm) in basal diameter by 1992. Oriental bittersweet did not invade sites where common greenbrier dominated the initial community [34].
In Connecticut, Goslee [44] reported that Oriental bittersweet, and to a lesser extent, Japanese honeysuckle and grape (Vitis spp.), had invaded an old field, creating a liana-dominated field that excluded other woody species. This probably "dramatically altered" the successional path the old field would otherwise have followed [44].
Celastrus orbiculatus is a woody vine of the family Celastraceae.[1] It is commonly called Oriental bittersweet,[2][3][4] as well as Chinese bittersweet,[3] Asian bittersweet,[4] round-leaved bittersweet,[4] and Asiatic bittersweet. It is native to China, where it is the most widely distributed Celastrus species, and to Japan and Korea.[5] It was introduced into North America in 1879,[6] and is considered to be an invasive species in eastern North America.[7] It closely resembles the native North American species, Celastrus scandens, with which it will readily hybridize.[8]
The defining characteristic of the plant is its vines: they are thin, spindly, and have silver to reddish brown bark. They are generally between 1 and 4 cm (0.4 and 1.6 in) in diameter. However, if growth is not disturbed, vines can exceed 10 cm (3.9 in) and when cut, will show age rings that can exceed 20 years. When Celastrus orbiculatus grows by itself, it forms thickets; when it is near a tree the vines twist themselves around the trunk as high as 40 feet. The encircling vines have been known to strangle the host tree to death or break branches from the excess weight, which is also true of the slower-growing American species, C. scandens. The leaves are round and glossy, 2–12 cm (0.8–4.7 in) long, have toothed margins and grow in alternate patterns along the vines. Small green flowers produce distinctive red seeds which are encased in yellow pods that break open during autumn. All parts of the plant are poisonous.[9]
Due to systematic disturbances to eastern forests for wood production and recreation, Oriental bittersweet has naturalized to landscapes, roadsides, and woodlands of eastern North America. In the United States, it can be found as far south as Louisiana, as far north as Maine, and as far west as the Rocky Mountains.[10][11] It prefers mesic woods, where it has been known to eclipse native plants.[12]
Celastrus orbiculatus is cultivated as an ornamental plant. In the UK, it has gained the Royal Horticultural Society's Award of Garden Merit.[13]
Oriental bittersweet is a strong competitor in its environment, and its dispersal has endangered the survival of several other species. One attribute that contributes to the success of this species is having attractively colored fruit. As a result, it is eaten by mammals and birds, which excrete the seeds to different locations.
The introduction of Oriental bittersweet into new areas threatens the local flora because the native plants then have a strong competitor in the vicinity. The species is native to Eastern Asia, but was introduced to the US for aesthetic purposes.[14] It has been used in floral arrangements, and because of improper disposal the plant has been recklessly introduced into areas, affecting the ecology of over 33 states from Georgia to Wisconsin, and parts of the Appalachians.[14] The organism grows primarily in the perimeter of highly vegetative areas, allowing it to readily access the frontier of resources. Oriental bittersweet's ability to grow in a variety of environments has proven to be detrimental to many plant species along the Appalachian mountains and is moving more towards the West as time progresses.[15][16][17]
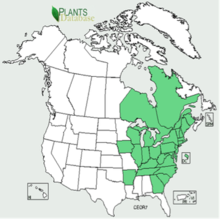
Oriental bittersweet employs multiple invasive and dispersal strategies allowing it to outcompete the surrounding plant species in non-native regions. This is a strong reason why the control of the species presents difficulties to manage.[18] The plant's invasion has created diverse ecological, managerial, and agricultural complications making it a focus of environmental conservation efforts.
Oriental bittersweet can be found growing in areas that are high and steep. When placed in 10 different sites with varying light intensity and nitrogen concentration, Oriental bittersweet was found to have higher aboveground biomass as well as a lower mortality rate in comparison to its congener species, Celastrus scandens (American bittersweet). This species is able to outcompete other species by more effectively responding to abiotic conditions such as sunlight. In diverse abiotic conditions (such as varying sunlight intensity and nitrogen concentrations), Oriental bittersweet has a mortality rate of 14% in comparison to the American bittersweet, which has a mortality rate of 33%.[19] Oriental bittersweet cannot thrive as efficiently when placed in extremely wet and dry environments; however, it flourishes in moderate rainfall environments which leads to an increased growth rate.
Sunlight is one of the most vital resources for Oriental bittersweet. As demonstrated by controlled experiments, Oriental bittersweet grows more rapidly in environments that fare a higher amount of sunlight. In a study where populations received above 28% sunlight, it exhibited a higher amount of growth and biomass.[19] This study used layers of woven cloth to control the percentage of available sunlight. In this experiment, the TLL ratio (the living length of stems on each plant) increased when Oriental bittersweet was exposed to higher amounts of sunlight.[19] If Oriental bittersweet was exposed to 2% sunlight, then the TLL ratio decreased.[19] Oriental bittersweet can increase in biomass by 20% when exposed to 28% sunlight rather than 2%. The plant's strong response to sunlight parallels its role as an invasive species, as it can outcompete other species by fighting for and receiving more sunlight. Although growth ratios decrease when Oriental bittersweet is exposed to 2% sunlight (due to a decrease in photosynthetic ability), it still exhibited a 90% survival rate.[20] Experimental data has indicated that Oriental bittersweet has a strong ability to tolerate low light conditions "ranging on average from 0.8 to 6.4% transmittance".[21] In comparison to its congener American bittersweet, when placed in habitats with little light, Oriental bittersweet was found to have increased height, increased aboveground biomass, and increased total leaf mass.[20][21] Oriental bittersweet, in comparison to many other competing species, is the better competitor in attaining sunlight.
Temperature is another variable that plays a role in Oriental bittersweet's growth and development as an invasive species. Unlike other invasive species, high summer temperatures have been shown to inhibit plant growth. Oriental bittersweet has also been shown to be positively favored in habitats experiencing high annual precipitation. This is noteworthy as it contrasts sharply with other common invasive species such as Berberis thunbergii and Euonymus alatus which have been shown to have a decreased probability of establishment when placed in environments experiencing high annual precipitation.[22]
Compared to other invasive species analyzed in a recent study, Oriental bittersweet was more prevalent in landscapes dominated by developed areas.[22] Open and abandoned habitats were also found to positively influence the spread of the plant compared to other invasive species.[22] Additionally the species is heavily favored in edge habitats. This ability to live in various environmental conditions raises the concern of the plant's dispersal.
A determining factor regarding Oriental bittersweet's ability to outcompete native plant species is its ability to form mutualistic associations with mycorrhizal fungi, specifically arbuscular mycorrhizal fungi.[23] Oriental bittersweet growth is highly dependent on the absorption of phosphorus. In a recent study, growth was found to be greater when arbuscular mycorrhizal fungi were present in soil with low phosphorus concentrations, compared to when the plant was placed in an environment with high soil phosphorus concentrations with no arbuscular mycorrhizal fungi were present.[23] The results from this study show the importance of symbiotic relationships in allowing Oriental bittersweet to effectively uptake nutrients from its surroundings. Additionally, the symbiotic relationship with mycorrhizae allows this invasive species to utilize less of its energy in root biomass to absorb necessary nutrients. This may be crucial in allowing Oriental bittersweet to act as an effective invasive species as it is able to allocate more energy to its aboveground biomass instead of its belowground biomass; a significant point regarding this plant's invasiveness relies on photosynthetic ability and reproductive capacity.[23] The symbiotic relationship established with fungi only occurs with arbuscular mycorrhizal fungi, while no such relationship has been observed with ectomycorrhizal fungi. These studies have shown that suitable mycorrhizae are a strong determining factor regarding whether a plant can survive in its environment.[23] Studies have also shown evidence that “introduced plant species can modify microbial communities in the soil surrounding not only their own roots, but also the roots of neighboring plants, thereby altering competitive interactions among the plant species”.[23] This may be a key invasive trait for Oriental bittersweet, as it allows the plant to negatively affect surrounding plant life by altering their underground symbiotic microbial relationships.[23] However, further experimentation is necessary to determine whether this organism employs this trait as an invasive strategy.
One of Oriental bittersweet's invasive characteristics is its effective utilization of energy to increase plant height, thus giving it a competitive advantage over similar plants. A study conducted in 2006 showed that, in comparison to its congener American bittersweet, Oriental bittersweet had increased height, increased aboveground biomass, and increased total leaf mass.[20] This is not to say that Oriental bittersweet outperformed American bittersweet in all criteria: in comparison to Oriental bittersweet, “American bittersweet had increased stem diameter, single leaf area, and leaf mass to stem mass ratio,” suggestive that American bittersweet focused growth on ulterior portions of the plant rather than plant characteristics emphasized by Oriental bittersweet such as stem length.[20] This is significant as height plays a major role in allowing Oriental bittersweet to outcompete surrounding vegetation.[20] Focusing growth on stem length allows it to be in a strong position to absorb light, while also negatively impacting surrounding plant life by creating shade-like conditions.
The species' vine-like morphology has also been shown to have negative effects on surrounding plant life. For example, evidence suggests that this morphological characteristic facilitates its ability to girdle nearby trees, creating an overall negative effect on the trees such as making them more susceptible to ice damage or damaging branches due to the weight of the plant.[24] Additionally, studies have suggested that Oriental bittersweet is capable of siphoning away nutrients from surrounding plants. The study found this to occur in a variety of environments, suggestive of both the plant's increased relative plasticity as well as increased nutrient uptake.[21]
One study observed that the presence of Oriental bittersweet increases the alkalinity of the surrounding soil, a characteristic of many successful invasive plant species.[24] This alters the availability of essential nutrients and hinders the nutrient uptake ability of native plants. Though the relationship between Oriental bittersweet and the alkalinity of the soil is consistent, there are a number of proposed mechanisms for this observation. The plant's significant above-ground biomass demands the preferential uptake of nitrate over ammonia, leading to soil nitrification. It also has a high cation-exchange capacity, which also supports the larger biomass. Either of these functions could explain the increased alkalinity, but further experimentation is needed to pinpoint the exact mechanism.[24]
Another major threat posed by Oriental bittersweet is hybridization with American bittersweet. Hybridization occurs readily between American bittersweet females and Oriental bittersweet males, though the opposite is known to occur to a lesser extent. The resulting hybrid species is fully capable of reproduction.[25] In theory, if the Oriental bittersweet invasion continues to worsen, widespread hybridization could genetically disrupt the entire American bittersweet population, possibly rendering it extinct.[15]
To minimize the effects of Oriental bittersweet's invasion into North American habitats, its growth and dispersal must be tightly managed. Early detection is essential for successful conservation efforts. To reduce further growth and dispersal, above-ground vegetation is cut and any foliage is sprayed with triclopyr, a common herbicide. Glyphosate is another chemical method of control. These two herbicides are usually sprayed directly on the plants in late fall to prevent other plants from being targeted. These steps must be repeated annually, or whenever regrowth is observed.[26] Triclopyr is non-toxic to most animal and insect species and slightly toxic to some species of fish, but it has a half-life of less than a day in water, making it safe and effective for field use.[26][27] Mechanical methods have also been used, but they are not as effective due to the difficulty of completely removing the root.[28] There is also no biological control agent available in helping control this species.[29] Mechanical and chemical methods are being used, but they are only temporarily fixing the situation.
Bicelaphanol A is a neuroprotective dimeric-trinorditerpene isolated from the bark of Celastrus orbiculatus.[30]
Despite the modest toxicity of its fruit, some livestock browse on the leaves without effect. Its vines, which are durable and tough, are a good source of weaving material for baskets. The fibrous inner bark can be used to make strong cordage.
{{cite web}}: CS1 maint: multiple names: authors list (link) {{cite web}}: CS1 maint: multiple names: authors list (link) Celastrus orbiculatus is a woody vine of the family Celastraceae. It is commonly called Oriental bittersweet, as well as Chinese bittersweet, Asian bittersweet, round-leaved bittersweet, and Asiatic bittersweet. It is native to China, where it is the most widely distributed Celastrus species, and to Japan and Korea. It was introduced into North America in 1879, and is considered to be an invasive species in eastern North America. It closely resembles the native North American species, Celastrus scandens, with which it will readily hybridize.